当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxime Ligation via in situ Oxidation of N-Phenylglycinyl Peptides
Organic Letters ( IF 4.9 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1021/acs.orglett.8b00713 Quibria A. E. Guthrie 1 , Caroline Proulx 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1021/acs.orglett.8b00713 Quibria A. E. Guthrie 1 , Caroline Proulx 1
Affiliation
|
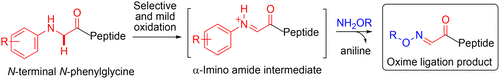
Mild conditions for oxime ligations via in situ generation of α-imino amide intermediates are reported. The evaluation of a variety of N-terminal N-phenylglycine residues revealed that a metal-free, chemoselective oxidation was possible using oxygen as the only oxidant in buffer at pH 7.0. Moreover, selective unmasking of an inert residue by addition of potassium ferricyanide is demonstrated. These simple and mild conditions, which can be fine-tuned by the electronic properties of the N-phenylglycine residue, offer unique advantages over conventional approaches for oxime ligations.
中文翻译:

N-苯基甘氨酰肽的原位氧化肟连接
报道了通过原位产生α-亚氨基酰胺中间体进行肟连接的温和条件。对各种N-末端N-苯基甘氨酸残基的评估表明,使用氧气作为pH 7.0缓冲液中的唯一氧化剂,可以进行无金属的化学选择性氧化。另外,还证明了通过添加铁氰化钾选择性地使惰性残基不被掩盖。这些简单温和的条件可以通过N-苯基甘氨酸残基的电子特性进行微调,与传统的肟连接方法相比,具有独特的优势。
更新日期:2018-04-25
中文翻译:

N-苯基甘氨酰肽的原位氧化肟连接
报道了通过原位产生α-亚氨基酰胺中间体进行肟连接的温和条件。对各种N-末端N-苯基甘氨酸残基的评估表明,使用氧气作为pH 7.0缓冲液中的唯一氧化剂,可以进行无金属的化学选择性氧化。另外,还证明了通过添加铁氰化钾选择性地使惰性残基不被掩盖。这些简单温和的条件可以通过N-苯基甘氨酸残基的电子特性进行微调,与传统的肟连接方法相比,具有独特的优势。































 京公网安备 11010802027423号
京公网安备 11010802027423号