当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-functional carbon dot-labeled heavy-chain ferritin for self-targeting bio-imaging and chemo-photodynamic therapy
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-04-23 00:00:00 , DOI: 10.1039/c8tb00118a Hanchun Yao 1, 2, 3, 4, 5 , Weiwei Zhao 1, 2, 3, 4 , Suge Zhang 1, 2, 3, 4 , Xiaofang Guo 1, 2, 3, 4 , Ying Li 1, 2, 3, 4 , Bin Du 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2018-04-23 00:00:00 , DOI: 10.1039/c8tb00118a Hanchun Yao 1, 2, 3, 4, 5 , Weiwei Zhao 1, 2, 3, 4 , Suge Zhang 1, 2, 3, 4 , Xiaofang Guo 1, 2, 3, 4 , Ying Li 1, 2, 3, 4 , Bin Du 1, 2, 3, 4, 5
Affiliation
|
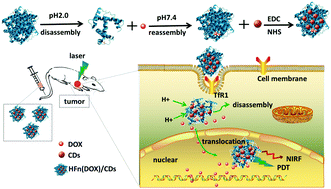
Image-guided cancer nanotheranostics with a simple nano-platform are seriously significant for nanomedicine. In this study, a novel design is described to achieve sensitive bio-imaging and effective treatment by utilizing heavy-chain ferritin (HFn) nanocages as a vector coupled with dual-functional carbon dots (CDs) on the surface of ferritin and encapsulating the chemotherapeutic drug doxorubicin (DOX). The CDs obtained herein emit bright fluorescence in the red region, which can be applied to bio-imaging in vivo. More significantly, the CDs can produce reactive oxygen species (ROS) under laser irradiation at 532 nm and cause damage to the DNA in the nucleus. These unique properties enabled CDs to act as a theranostic agent. Owing to the self-targeting ability of HFn, the final nanoparticles can internalize into cancer cells more efficiently. The nanoparticles can translocate into the nucleus after DNA damage resulting from the partial release of DOX into the cytoplasm, thereby increasing the nuclear delivery of the drug. The results of this study indicate that the multifunctional HFn(DOX)/CD nanoparticles have potential as a clinically available cancer theranostic agent to deliver diagnostic agents and therapeutic drugs into the cancer cells and thus provide a noninvasive, highly sensitive imaging approach and guidance to the chemo-photodynamic therapy simultaneously.
中文翻译:

双功能碳点标记的重链铁蛋白,用于自靶向生物成像和化学光动力疗法
具有简单纳米平台的图像引导的癌症纳米治疗对于纳米医学而言意义重大。在这项研究中,描述了一种新颖的设计,该技术通过利用重链铁蛋白(HFn)纳米笼作为载体与铁蛋白表面上的双功能碳点(CD)偶联并封装化学疗法来实现敏感的生物成像和有效治疗药物阿霉素(DOX)。本文获得的CD在红色区域发出明亮的荧光,可将其应用于体内生物成像。更重要的是,CD可以在532 nm的激光照射下产生活性氧(ROS),并破坏细胞核中的DNA。这些独特的属性使CD可以用作诊断治疗剂。由于HFn的自我靶向能力,最终的纳米颗粒可以更有效地内化到癌细胞中。由于DOX部分释放到细胞质中而导致DNA损伤后,纳米颗粒可以转移到细胞核中,从而增加了药物的核传递。这项研究的结果表明,多功能HFn(DOX)/ CD纳米颗粒具有作为临床可用的癌症治疗剂的潜力,可以将诊断剂和治疗药物输送到癌细胞中,从而提供无创,
更新日期:2018-04-23
中文翻译:

双功能碳点标记的重链铁蛋白,用于自靶向生物成像和化学光动力疗法
具有简单纳米平台的图像引导的癌症纳米治疗对于纳米医学而言意义重大。在这项研究中,描述了一种新颖的设计,该技术通过利用重链铁蛋白(HFn)纳米笼作为载体与铁蛋白表面上的双功能碳点(CD)偶联并封装化学疗法来实现敏感的生物成像和有效治疗药物阿霉素(DOX)。本文获得的CD在红色区域发出明亮的荧光,可将其应用于体内生物成像。更重要的是,CD可以在532 nm的激光照射下产生活性氧(ROS),并破坏细胞核中的DNA。这些独特的属性使CD可以用作诊断治疗剂。由于HFn的自我靶向能力,最终的纳米颗粒可以更有效地内化到癌细胞中。由于DOX部分释放到细胞质中而导致DNA损伤后,纳米颗粒可以转移到细胞核中,从而增加了药物的核传递。这项研究的结果表明,多功能HFn(DOX)/ CD纳米颗粒具有作为临床可用的癌症治疗剂的潜力,可以将诊断剂和治疗药物输送到癌细胞中,从而提供无创,






























 京公网安备 11010802027423号
京公网安备 11010802027423号