当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of the Solubility, Supersolubility and Metastable Zone Width of Li2CO3 in the LiCl–NaCl–KCl–Na2SO4 System from 293.15 to 353.15K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-04-23 , DOI: 10.1021/acs.jced.7b01012 Huaiyou Wang 1, 2 , Baoqiang Du 1, 2 , Min Wang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-04-23 , DOI: 10.1021/acs.jced.7b01012 Huaiyou Wang 1, 2 , Baoqiang Du 1, 2 , Min Wang 1, 2
Affiliation
|
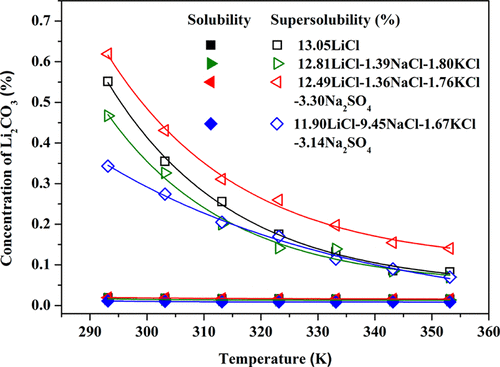
Isothermal dissolution method and turbidity technology were employed to measure the solubility, the supersolubility, and the metastable zone width of Li2CO3 in mixed LiCl–NaCl–KCl–Na2SO4 solutions at temperatures ranging from 293.15 to 353.15 K. It was found that the solubility values of Li2CO3 increased as the Na2SO4 concentration increased and decreased as the NaCl and KCl concentrations increased. In addition, these two opposite effects both affected the solubility of Li2CO3 in mixed electrolyte solutions. NaCl and KCl impurities decreased Li2CO3 supersolubility, which could be overcome by adding a small amount of Na2SO4 salt. The influence of the impurities on the metastable zone width differed from their influence on the solubility. The metastable zone width in electrolyte solutions was mainly affected by Na2SO4 salt, where it increased with the increasing Na2SO4 concentration. Meanwhile, NaCl and KCl salts had little impact on the metastable zone width. Overall, this study provides the basic data for the crystallization process of the lithium carbonate from salt lake brines in the industrial production process.
中文翻译:

LiCl–NaCl–KCl–Na 2 SO 4体系中Li 2 CO 3在293.15至353.15K范围内的溶解度,超溶解度和亚稳区域宽度的研究
采用等温溶解度法和浊度法测量了Li 2 CO 3在293.15至353.15 K范围内的混合LiCl–NaCl–KCl–Na 2 SO 4溶液中的溶解度,超溶解度和亚稳区域宽度。发现Li 2 CO 3的溶解度值随Na 2 SO 4浓度的增加而增加,随NaCl和KCl浓度的增加而减小。另外,这两种相反的作用都影响了Li 2 CO 3在混合电解质溶液中的溶解度。NaCl和KCl杂质降低Li 2 CO3超溶解性,可以通过添加少量Na 2 SO 4盐来克服。杂质对亚稳区域宽度的影响与其对溶解度的影响不同。电解质溶液中亚稳区的宽度主要受Na 2 SO 4盐的影响,并随Na 2 SO 4浓度的增加而增加。同时,NaCl和KCl盐对亚稳区的宽度影响很小。总的来说,这项研究为工业生产过程中盐湖盐水中碳酸锂的结晶过程提供了基础数据。
更新日期:2018-04-24
中文翻译:

LiCl–NaCl–KCl–Na 2 SO 4体系中Li 2 CO 3在293.15至353.15K范围内的溶解度,超溶解度和亚稳区域宽度的研究
采用等温溶解度法和浊度法测量了Li 2 CO 3在293.15至353.15 K范围内的混合LiCl–NaCl–KCl–Na 2 SO 4溶液中的溶解度,超溶解度和亚稳区域宽度。发现Li 2 CO 3的溶解度值随Na 2 SO 4浓度的增加而增加,随NaCl和KCl浓度的增加而减小。另外,这两种相反的作用都影响了Li 2 CO 3在混合电解质溶液中的溶解度。NaCl和KCl杂质降低Li 2 CO3超溶解性,可以通过添加少量Na 2 SO 4盐来克服。杂质对亚稳区域宽度的影响与其对溶解度的影响不同。电解质溶液中亚稳区的宽度主要受Na 2 SO 4盐的影响,并随Na 2 SO 4浓度的增加而增加。同时,NaCl和KCl盐对亚稳区的宽度影响很小。总的来说,这项研究为工业生产过程中盐湖盐水中碳酸锂的结晶过程提供了基础数据。















































 京公网安备 11010802027423号
京公网安备 11010802027423号