PLoS Pathogens ( IF 5.5 ) Pub Date : 2018-04-23 , DOI: 10.1371/journal.ppat.1006998 Dustin J. Little , Roland Pfoh , François Le Mauff , Natalie C. Bamford , Christina Notte , Perrin Baker , Manita Guragain , Howard Robinson , Gerald B. Pier , Mark Nitz , Rajendar Deora , Donald C. Sheppard , P. Lynne Howell
|
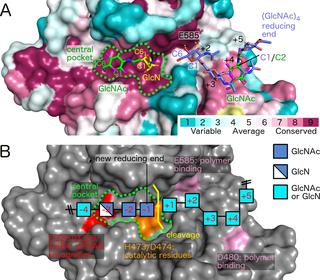
Poly-β(1,6)-N-acetyl-D-glucosamine (PNAG) is a major biofilm component of many pathogenic bacteria. The production, modification, and export of PNAG in Escherichia coli and Bordetella species require the protein products encoded by the pgaABCD operon. PgaB is a two-domain periplasmic protein that contains an N-terminal deacetylase domain and a C-terminal PNAG binding domain that is critical for export. However, the exact function of the PgaB C-terminal domain remains unclear. Herein, we show that the C-terminal domains of Bordetella bronchiseptica PgaB (PgaBBb) and E. coli PgaB (PgaBEc) function as glycoside hydrolases. These enzymes hydrolyze purified deacetylated PNAG (dPNAG) from Staphylococcus aureus, disrupt PNAG-dependent biofilms formed by Bordetella pertussis, Staphylococcus carnosus, Staphylococcus epidermidis, and E. coli, and potentiate bacterial killing by gentamicin. Furthermore, we found that PgaBBb was only able to hydrolyze PNAG produced in situ by the E. coli PgaCD synthase complex when an active deacetylase domain was present. Mass spectrometry analysis of the PgaB-hydrolyzed dPNAG substrate showed a GlcN-GlcNAc-GlcNAc motif at the new reducing end of detected fragments. Our 1.76 Å structure of the C-terminal domain of PgaBBb reveals a central cavity within an elongated surface groove that appears ideally suited to recognize the GlcN-GlcNAc-GlcNAc motif. The structure, in conjunction with molecular modeling and site directed mutagenesis led to the identification of the dPNAG binding subsites and D474 as the probable catalytic acid. This work expands the role of PgaB within the PNAG biosynthesis machinery, defines a new glycoside hydrolase family GH153, and identifies PgaB as a possible therapeutic agent for treating PNAG-dependent biofilm infections.
中文翻译:

PgaB直向同源物包含一个糖苷水解酶结构域,该结构域可切割脱乙酰基的聚-β(1,6)-N-乙酰基葡糖胺,并可以破坏细菌生物膜
聚-β(1,6)-N-乙酰基-D-葡萄糖胺(PNAG)是许多致病细菌的主要生物膜成分。PNAG在大肠杆菌和博德特氏菌物种中的生产,修饰和出口需要pgaABCD操纵子编码的蛋白质产物。PgaB是一个两结构域周质蛋白,包含一个N端脱乙酰基酶结构域和一个C端PNAG结合结构域,这对输出至关重要。但是,PgaB C末端域的确切功能仍不清楚。在这里,我们显示了支气管炎博德特氏菌PgaB(PgaB Bb)和E的C末端结构域。大肠杆菌PgaB(PgaB Ec)起糖苷水解酶的作用。这些酶水解纯化的脱乙酰化PNAG从(dPNAG)金黄色葡萄球菌,破坏由形成PNAG依赖性生物膜百日咳博德特氏菌,肉葡萄球菌,表皮葡萄球菌,和ë。杆菌,并增强庆大霉素的杀菌作用。此外,我们发现,PgaB了Bb只能够产生水解PNAG原位由Ë。大肠杆菌存在活性脱乙酰基酶结构域时,PgaCD合酶复合物。PgaB水解的dPNAG底物的质谱分析显示,在检测到的片段的新还原端处有一个GlcN-GlcNAc-GlcNAc基序。我们的PgaB Bb C末端结构域的1.76Å结构揭示了一个细长的表面凹槽内的中央空腔,看起来非常适合识别GlcN-GlcNAc-GlcNAc基序。该结构,结合分子建模和定点诱变,导致鉴定了dPNAG结合亚位点和D474作为可能的催化酸。这项工作扩大了PgaB在PNAG生物合成机制中的作用,定义了一个新的糖苷水解酶家族GH153,并确定了PgaB作为治疗PNAG依赖性生物膜感染的可能治疗剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号