当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein semisynthesis provides access to Tau disease-associated post-translational modifications (PTMs) and paves the way to deciphering the Tau PTM code in health and diseased states
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-23 , DOI: 10.1021/jacs.8b02668 Mahmood Haj-Yahya 1 , Hilal A. Lashuel 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-23 , DOI: 10.1021/jacs.8b02668 Mahmood Haj-Yahya 1 , Hilal A. Lashuel 1
Affiliation
|
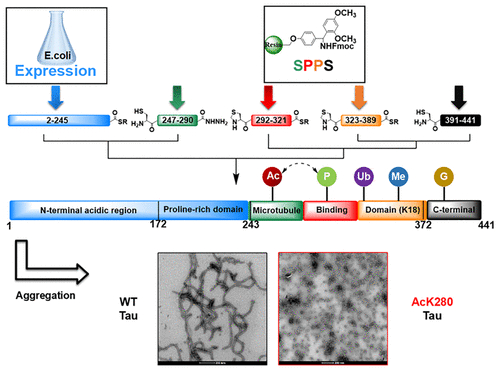
The microtubule-associated protein Tau plays a central role in neurodegeneration and is a leading therapeutic target for the treatment of Alzheimer's disease (AD). Several lines of evidence suggest that post-translational modifications (PTMs) regulate the function(s) of Tau, including its subcellular localization, clearance, aggregation, toxicity, and pathological spreading. However, the lack of tools and methodologies that allow site-specific introduction of PTMs in Tau have limited our ability to dissect the role of PTMs in regulating Tau functions in health and disease. To facilitate deciphering the Tau PTM code, we have developed, for the first time, semisynthetic strategies that allow for the site-specific introduction of single or multiple physiological or disease-associated PTMs that occur within residues 246-441 of Tau, which includes the microtubule-binding domain (MTBD). As a proof of concept, we produced unmodified Tau and three Tau variants with single or multiple disease-associated PTMs that were not previously accessible as homogeneously modified proteins, AcK280, pY310, and pS396/pS404. We then focused on investigating the effect of acetylation at lysine 280 (AcK280) on the structure, aggregation, and microtubule binding properties of Tau. Our results show that site-specific acetylation at K280 significantly enhances the aggregation rate of Tau and impairs microtubule assembly. Surprisingly, compared with unmodified Tau, which forms long and flexible filaments, AcK280 Tau forms predominantly globular oligomers and short fibrils (<200 nm) that exhibit a reduced propensity to assemble into long filaments. These findings are consistent with the increased aggregation propensity and pathogenicity of this mutant in animal models of AD and suggest that acetylation at this residue might enhance the seeding capacity or formation of toxic Tau species in vivo. Beyond acetylation and phosphorylation, the development of this semisynthetic strategy provides new opportunities to investigate other types of Tau PTMs and to study the cross-talk between PTMs that occurs within residues 246-441, which were previously inaccessible, thereby paving the way to deciphering the Tau PTM code in health and disease.
中文翻译:

蛋白质半合成提供了对 Tau 疾病相关的翻译后修饰 (PTM) 的访问,并为破译健康和患病状态下的 Tau PTM 代码铺平了道路
微管相关蛋白 Tau 在神经退行性疾病中起着核心作用,是治疗阿尔茨海默病 (AD) 的主要治疗靶点。多项证据表明,翻译后修饰 (PTM) 可调节 Tau 的功能,包括其亚细胞定位、清除、聚集、毒性和病理扩散。然而,由于缺乏允许特定地点在 Tau 中引入 PTM 的工具和方法,我们无法剖析 PTM 在调节健康和疾病中的 Tau 功能中的作用。为了便于破译 Tau PTM 代码,我们首次开发了半合成策略,允许在 Tau 的残基 246-441 内特定位置引入单个或多个生理或疾病相关的 PTM,其中包括微管结合域 (MTBD)。作为概念证明,我们生产了未修饰的 Tau 和三个带有单个或多个疾病相关 PTM 的 Tau 变体,这些 PTM 以前无法作为均质修饰的蛋白质 AcK280、pY310 和 pS396/pS404 获得。然后,我们专注于研究赖氨酸 280 (AcK280) 乙酰化对 Tau 的结构、聚集和微管结合特性的影响。我们的结果表明,K280 的位点特异性乙酰化显着提高了 Tau 的聚集率并损害了微管组装。令人惊讶的是,与形成长而柔韧的细丝的未修饰 Tau 相比,AcK280 Tau 主要形成球状低聚物和短原纤维 (<200 nm),它们组装成长丝的倾向降低。这些发现与 AD 动物模型中该突变体增加的聚集倾向和致病性一致,并表明该残基的乙酰化可能增强体内接种能力或有毒 Tau 物种的形成。除了乙酰化和磷酸化之外,这种半合成策略的发展为研究其他类型的 Tau PTM 和研究发生在残基 246-441 内的 PTM 之间的串扰提供了新的机会,这是以前无法访问的,从而为破译 Tau PTM 铺平了道路。健康和疾病中的 Tau PTM 代码。
更新日期:2018-04-23
中文翻译:

蛋白质半合成提供了对 Tau 疾病相关的翻译后修饰 (PTM) 的访问,并为破译健康和患病状态下的 Tau PTM 代码铺平了道路
微管相关蛋白 Tau 在神经退行性疾病中起着核心作用,是治疗阿尔茨海默病 (AD) 的主要治疗靶点。多项证据表明,翻译后修饰 (PTM) 可调节 Tau 的功能,包括其亚细胞定位、清除、聚集、毒性和病理扩散。然而,由于缺乏允许特定地点在 Tau 中引入 PTM 的工具和方法,我们无法剖析 PTM 在调节健康和疾病中的 Tau 功能中的作用。为了便于破译 Tau PTM 代码,我们首次开发了半合成策略,允许在 Tau 的残基 246-441 内特定位置引入单个或多个生理或疾病相关的 PTM,其中包括微管结合域 (MTBD)。作为概念证明,我们生产了未修饰的 Tau 和三个带有单个或多个疾病相关 PTM 的 Tau 变体,这些 PTM 以前无法作为均质修饰的蛋白质 AcK280、pY310 和 pS396/pS404 获得。然后,我们专注于研究赖氨酸 280 (AcK280) 乙酰化对 Tau 的结构、聚集和微管结合特性的影响。我们的结果表明,K280 的位点特异性乙酰化显着提高了 Tau 的聚集率并损害了微管组装。令人惊讶的是,与形成长而柔韧的细丝的未修饰 Tau 相比,AcK280 Tau 主要形成球状低聚物和短原纤维 (<200 nm),它们组装成长丝的倾向降低。这些发现与 AD 动物模型中该突变体增加的聚集倾向和致病性一致,并表明该残基的乙酰化可能增强体内接种能力或有毒 Tau 物种的形成。除了乙酰化和磷酸化之外,这种半合成策略的发展为研究其他类型的 Tau PTM 和研究发生在残基 246-441 内的 PTM 之间的串扰提供了新的机会,这是以前无法访问的,从而为破译 Tau PTM 铺平了道路。健康和疾病中的 Tau PTM 代码。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号