当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CHF3–CHClF2 Binary Competitive Adsorption Equilibria in Graphitic Slit Pores: Monte Carlo Simulations and Breakthrough Curve Experiments
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-25 , DOI: 10.1021/acs.iecr.8b00141
Qiang Fu 1 , Hideki Tanaka 2 , Minoru T. Miyahara 2 , Yingjie Qin 1 , Yuanhui Shen 1 , Donghui Zhang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-25 , DOI: 10.1021/acs.iecr.8b00141
Qiang Fu 1 , Hideki Tanaka 2 , Minoru T. Miyahara 2 , Yingjie Qin 1 , Yuanhui Shen 1 , Donghui Zhang 1
Affiliation
|
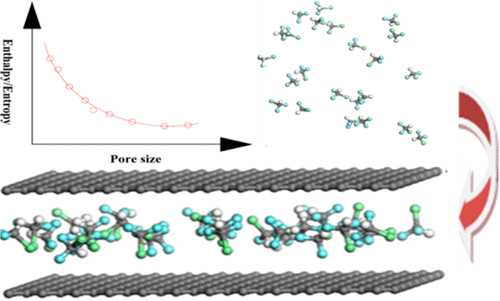
The separation and recovery of the greenhouse gases CHF3 and CHClF2 from emissions is essential to avoid adverse environmental effects. Nanoporous carbons are excellent candidates for the capture of CHF3. However, little research has been done on the effect of pore size on the adsorption capacity and selectivity of CHClF2 over CHF3 in nanoporous carbon materials. The effect of the pore size on the competitive adsorption of a binary CHF3–CHClF2 mixture in graphitic slit pores has been investigated using a combination of Monte Carlo simulations and breakthrough curve experiments over a wide range of pressures and temperatures. The entropy changes have a considerable effect on selectivity of CHClF2 over CHF3 because the size of the adsorbate molecule is close to the effective height of the pores; conversely, adsorption energy has a significant impact. The selectivity of CHClF2 over CHF3 at 0.05–1.0 MPa increases in the order 19.47 < 16.62 < 13.20 < 11.77 < 8.30 < 5.17 < 5.67 Å. The mechanisms for adsorption and the selectivity of CHClF2 over CHF3 were determined, as were the effects of pore size and pressure on adsorption energy and adsorption entropy. This combination of methods provides an effective strategy for the design and screening of adsorbent materials for CHF3–CHClF2 separation and recovery applications.
中文翻译:

石墨狭缝中CHF 3 –CHClF 2的二元竞争吸附平衡:蒙特卡洛模拟和突围曲线实验
从排放中分离和回收温室气体CHF 3和CHClF 2对于避免不利的环境影响至关重要。纳米多孔碳是捕获CHF 3的极佳候选者。然而,关于纳米多孔碳材料中孔径对CHClF 2相对于CHF 3的吸附容量和选择性的影响的研究很少。孔径对二元CHF 3 –CHClF 2竞争性吸附的影响在广泛的压力和温度范围内,使用蒙特卡罗模拟和突破曲线实验相结合,研究了石墨狭缝孔中的混合气。熵的变化对CHClF 2相对于CHF 3的选择性有相当大的影响,因为被吸附物分子的大小接近孔的有效高度。相反,吸附能有很大的影响。在0.05–1.0 MPa下,CHClF 2对CHF 3的选择性以19.47 <16.62 <13.20 <11.77 <8.30 <5.17 <5.67Å的顺序增加。CHClF 2相对于CHF 3的吸附机理和选择性确定了孔径和压力对吸附能和吸附熵的影响。这种方法的组合为CHF 3 –CHClF 2分离和回收应用的吸附剂材料的设计和筛选提供了有效的策略。
更新日期:2018-04-26
中文翻译:

石墨狭缝中CHF 3 –CHClF 2的二元竞争吸附平衡:蒙特卡洛模拟和突围曲线实验
从排放中分离和回收温室气体CHF 3和CHClF 2对于避免不利的环境影响至关重要。纳米多孔碳是捕获CHF 3的极佳候选者。然而,关于纳米多孔碳材料中孔径对CHClF 2相对于CHF 3的吸附容量和选择性的影响的研究很少。孔径对二元CHF 3 –CHClF 2竞争性吸附的影响在广泛的压力和温度范围内,使用蒙特卡罗模拟和突破曲线实验相结合,研究了石墨狭缝孔中的混合气。熵的变化对CHClF 2相对于CHF 3的选择性有相当大的影响,因为被吸附物分子的大小接近孔的有效高度。相反,吸附能有很大的影响。在0.05–1.0 MPa下,CHClF 2对CHF 3的选择性以19.47 <16.62 <13.20 <11.77 <8.30 <5.17 <5.67Å的顺序增加。CHClF 2相对于CHF 3的吸附机理和选择性确定了孔径和压力对吸附能和吸附熵的影响。这种方法的组合为CHF 3 –CHClF 2分离和回收应用的吸附剂材料的设计和筛选提供了有效的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号