当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Understanding of Alloy Effect and Water Promotion for Pd-Cu Bimetallic Catalysts in CO2 Hydrogenation to Methanol
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acscatal.7b04150 Xiaowa Nie 1 , Xiao Jiang 2 , Haozhi Wang 1 , Wenjia Luo 3 , Michael J. Janik 4 , Yonggang Chen 5 , Xinwen Guo 1 , Chunshan Song 1, 2, 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acscatal.7b04150 Xiaowa Nie 1 , Xiao Jiang 2 , Haozhi Wang 1 , Wenjia Luo 3 , Michael J. Janik 4 , Yonggang Chen 5 , Xinwen Guo 1 , Chunshan Song 1, 2, 4
Affiliation
|
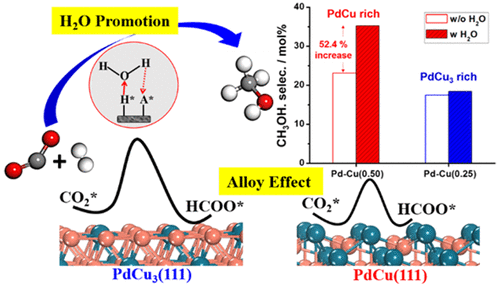
Density functional theory (DFT) calculations on Pd-Cu bimetallic catalysts reveal that the stepped PdCu(111) surface with coordinatively unsaturated Pd atoms exposed on the top is superior for CO2 and H2 activation and for CO2 hydrogenation to methanol in comparison to the flat Cu-rich PdCu3(111) surface. The energetically preferred path for CO2 to CH3OH over PdCu(111) proceeds through CO2* → HCOO* → HCOOH* → H2COOH* → CH2O* → CH3O* → CH3OH*. CO formation from CO2 via a reverse water-gas shift (RWGS) proceeds more quickly than CH3OH formation in terms of kinetic calculations, in line with experimental observation. A small amount of water, which is produced in situ from both RWGS and CH3OH formation, can accelerate CO2 conversion to methanol by reducing the kinetic barriers for O–H bond formation steps and enhancing the TOF. Water participation in the reaction alters the rate-limiting step according to the degree of rate control (DRC) analysis. In comparison to CO2, CO hydrogenation to methanol on PdCu(111) encounters higher barriers and thus is slower in kinetics. Complementary to the DFT results, CO2 hydrogenation experiments over SiO2-supported bimetallic catalysts show that the Pd-Cu(0.50) that is rich in a PdCu alloy phase is more selective to methanol than the PdCu3-rich Pd-Cu(0.25). Moreover, advanced CH3OH selectivity is also evidenced on Pd-Cu(0.50) at a specific water vapor concentration (0.03 mol %), whereas that of Pd-Cu(0.25) is not comparable. The present work clearly shows that the PdCu alloy surface structure has a major effect on the reaction pathway, and the presence of water can substantially influence the kinetics in CO2 hydrogenation to methanol.
中文翻译:

Pd-Cu双金属催化剂在CO 2加氢制甲醇中合金效应和助水机理的机理理解
在Pd-Cu双金属催化剂上的密度泛函理论(DFT)计算表明,与顶部相比,阶梯状PdCu(111)表面带有配位不饱和Pd原子暴露在顶部的CO 2和H 2活化以及CO 2加氢制甲醇均优于平坦的富铜PdCu 3(111)表面。相对于PdCu(111),CO 2转化为CH 3 OH的能量优选途径是通过CO 2 *→HCOO *→HCOOH *→H 2 COOH *→CH 2 O *→CH 3 O *→CH 3 OH *。通过反向水煤气变换(RWGS)由CO 2形成CO的过程比CH快得多在动力学计算方面,3 OH的形成与实验观察一致。RWGS和CH 3 OH的形成都少量产生水,通过减少O-H键形成步骤的动力学障碍并提高TOF ,可以加速CO 2转化为甲醇。根据速率控制(DRC)分析的程度,水参与反应会改变速率限制步骤。与CO 2相比,PdCu(111)上的CO加氢成甲醇遇到了更高的障碍,因此动力学较慢。作为DFT结果的补充,SiO 2上的CO 2加氢实验负载的双金属催化剂表明,与富含PdCu 3的Pd-Cu(0.25)相比,富含PdCu合金相的Pd-Cu(0.50)对甲醇的选择性更高。此外,在特定的水蒸气浓度(0.03 mol%)下,Pd-Cu(0.50)上的CH 3 OH选择性也得到了提高,而Pd-Cu(0.25)则没有可比性。本工作清楚地表明,PdCu合金的表面结构对反应路径有重大影响,并且水的存在会大大影响CO 2加氢制甲醇的动力学。
更新日期:2018-04-18
中文翻译:

Pd-Cu双金属催化剂在CO 2加氢制甲醇中合金效应和助水机理的机理理解
在Pd-Cu双金属催化剂上的密度泛函理论(DFT)计算表明,与顶部相比,阶梯状PdCu(111)表面带有配位不饱和Pd原子暴露在顶部的CO 2和H 2活化以及CO 2加氢制甲醇均优于平坦的富铜PdCu 3(111)表面。相对于PdCu(111),CO 2转化为CH 3 OH的能量优选途径是通过CO 2 *→HCOO *→HCOOH *→H 2 COOH *→CH 2 O *→CH 3 O *→CH 3 OH *。通过反向水煤气变换(RWGS)由CO 2形成CO的过程比CH快得多在动力学计算方面,3 OH的形成与实验观察一致。RWGS和CH 3 OH的形成都少量产生水,通过减少O-H键形成步骤的动力学障碍并提高TOF ,可以加速CO 2转化为甲醇。根据速率控制(DRC)分析的程度,水参与反应会改变速率限制步骤。与CO 2相比,PdCu(111)上的CO加氢成甲醇遇到了更高的障碍,因此动力学较慢。作为DFT结果的补充,SiO 2上的CO 2加氢实验负载的双金属催化剂表明,与富含PdCu 3的Pd-Cu(0.25)相比,富含PdCu合金相的Pd-Cu(0.50)对甲醇的选择性更高。此外,在特定的水蒸气浓度(0.03 mol%)下,Pd-Cu(0.50)上的CH 3 OH选择性也得到了提高,而Pd-Cu(0.25)则没有可比性。本工作清楚地表明,PdCu合金的表面结构对反应路径有重大影响,并且水的存在会大大影响CO 2加氢制甲醇的动力学。






























 京公网安备 11010802027423号
京公网安备 11010802027423号