当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Receptor-Mediated Delivery of CRISPR-Cas9 Endonuclease for Cell Type Specific Gene Editing
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-18 , DOI: 10.1021/jacs.8b01551 Romain Rouet , Benjamin A Thuma 1 , Marc D Roy 2 , Nathanael G Lintner 3 , David M Rubitski 2 , James E Finley 2 , Hanna M Wisniewska 1 , Rima Mendonsa , Ariana Hirsh , Lorena de Oñate , Joan Compte Barrón , Thomas J McLellan 1 , Justin Bellenger 1 , Xidong Feng 1 , Alison Varghese 1 , Boris A Chrunyk 1 , Kris Borzilleri 1 , Kevin D Hesp 1 , Kaihong Zhou , Nannan Ma , Meihua Tu 3 , Robert Dullea 4 , Kim F McClure 3 , Ross C Wilson , Spiros Liras 3 , Vincent Mascitti 1 , Jennifer A Doudna 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-04-18 , DOI: 10.1021/jacs.8b01551 Romain Rouet , Benjamin A Thuma 1 , Marc D Roy 2 , Nathanael G Lintner 3 , David M Rubitski 2 , James E Finley 2 , Hanna M Wisniewska 1 , Rima Mendonsa , Ariana Hirsh , Lorena de Oñate , Joan Compte Barrón , Thomas J McLellan 1 , Justin Bellenger 1 , Xidong Feng 1 , Alison Varghese 1 , Boris A Chrunyk 1 , Kris Borzilleri 1 , Kevin D Hesp 1 , Kaihong Zhou , Nannan Ma , Meihua Tu 3 , Robert Dullea 4 , Kim F McClure 3 , Ross C Wilson , Spiros Liras 3 , Vincent Mascitti 1 , Jennifer A Doudna 5
Affiliation
|
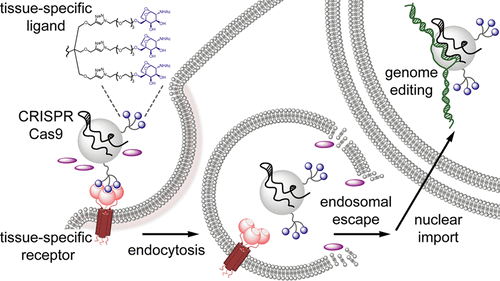
CRISPR-Cas RNA-guided endonucleases hold great promise for disrupting or correcting genomic sequences through site-specific DNA cleavage and repair. However, the lack of methods for cell- and tissue-selective delivery currently limits both research and clinical uses of these enzymes. We report the design and in vitro evaluation of S. pyogenes Cas9 proteins harboring asialoglycoprotein receptor ligands (ASGPrL). In particular, we demonstrate that the resulting ribonucleoproteins (Cas9-ASGPrL RNP) can be engineered to be preferentially internalized into cells expressing the corresponding receptor on their surface. Uptake of such fluorescently labeled proteins in liver-derived cell lines HEPG2 (ASGPr+) and SKHEP (control; diminished ASGPr) was studied by live cell imaging and demonstrates increased accumulation of Cas9-ASGPrL RNP in HEPG2 cells as a result of effective ASGPr-mediated endocytosis. When uptake occurred in the presence of a peptide with endosomolytic properties, we observed receptor-facilitated and cell-type specific gene editing that did not rely on electroporation or the use of transfection reagents. Overall, these in vitro results validate the receptor-mediated delivery of genome-editing enzymes as an approach for cell-selective gene editing and provide a framework for future potential applications to hepatoselective gene editing in vivo.
中文翻译:

受体介导的 CRISPR-Cas9 核酸内切酶递送用于细胞类型特异性基因编辑
CRISPR-Cas RNA 引导的核酸内切酶有望通过位点特异性 DNA 切割和修复来破坏或纠正基因组序列。然而,目前缺乏细胞和组织选择性递送的方法限制了这些酶的研究和临床应用。我们报告了带有脱唾液酸糖蛋白受体配体(ASGPrL)的化脓性链球菌Cas9蛋白的设计和体外评估。特别是,我们证明所产生的核糖核蛋白(Cas9-ASGPrL RNP)可以被设计为优先内化到在其表面表达相应受体的细胞中。通过活细胞成像研究了肝源性细胞系 HEPG2 (ASGPr+) 和 SKHEP(对照;ASGPr 减少)对此类荧光标记蛋白的摄取,结果表明,由于有效的 ASGPr 介导,HEPG2 细胞中 Cas9-ASGPrL RNP 的积累增加内吞作用。当在具有内体溶解特性的肽存在的情况下发生摄取时,我们观察到受体促进的和细胞类型特异性的基因编辑,其不依赖于电穿孔或转染试剂的使用。总体而言,这些体外结果验证了受体介导的基因组编辑酶递送作为细胞选择性基因编辑的一种方法,并为未来体内肝选择性基因编辑的潜在应用提供了框架。
更新日期:2018-04-18
中文翻译:

受体介导的 CRISPR-Cas9 核酸内切酶递送用于细胞类型特异性基因编辑
CRISPR-Cas RNA 引导的核酸内切酶有望通过位点特异性 DNA 切割和修复来破坏或纠正基因组序列。然而,目前缺乏细胞和组织选择性递送的方法限制了这些酶的研究和临床应用。我们报告了带有脱唾液酸糖蛋白受体配体(ASGPrL)的化脓性链球菌Cas9蛋白的设计和体外评估。特别是,我们证明所产生的核糖核蛋白(Cas9-ASGPrL RNP)可以被设计为优先内化到在其表面表达相应受体的细胞中。通过活细胞成像研究了肝源性细胞系 HEPG2 (ASGPr+) 和 SKHEP(对照;ASGPr 减少)对此类荧光标记蛋白的摄取,结果表明,由于有效的 ASGPr 介导,HEPG2 细胞中 Cas9-ASGPrL RNP 的积累增加内吞作用。当在具有内体溶解特性的肽存在的情况下发生摄取时,我们观察到受体促进的和细胞类型特异性的基因编辑,其不依赖于电穿孔或转染试剂的使用。总体而言,这些体外结果验证了受体介导的基因组编辑酶递送作为细胞选择性基因编辑的一种方法,并为未来体内肝选择性基因编辑的潜在应用提供了框架。































 京公网安备 11010802027423号
京公网安备 11010802027423号