当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Sensitive Water-Soluble Reversible Optical Probe for Hg2+ Detection
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00310 Sayani Das 1 , Anindita Sarkar 1 , Ananya Rakshit 1 , Ankona Datta 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-18 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00310 Sayani Das 1 , Anindita Sarkar 1 , Ananya Rakshit 1 , Ankona Datta 1
Affiliation
|
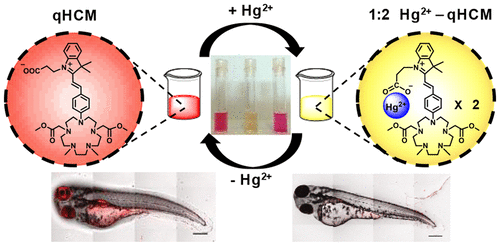
We report the serendipitous discovery of an optical mercury sensor while trying to develop a water-soluble manganese probe. The sensor is based on a pentaaza macrocycle conjugated to a hemicyanine dye. The pentaaza macrocycle earlier designed in our group was used to develop photoinduced electron transfer (PET)-based “turn-on” fluorescent sensors for manganese.(1) In an attempt to increase the water-solubility of the manganese sensors we changed the dye from BODIPY to hemicyanine. The resultant molecule qHCM afforded a distinct reversible change in the absorption features and a concomitant visible color change upon binding to Hg2+ ions, leading to a highly water-soluble mercury sensor with a 10 ppb detection limit. The molecule acts as a reversible “ON–OFF” fluorescent sensor for Hg2+ with a 35 times decrease in the emission intensity in the presence of 1 equiv of Hg2+ ions. We have demonstrated the applicability of the probe for detecting Hg2+ ions in living cells and in live zebrafish larvae using confocal fluorescence microscopy with visible excitation. High selectivity and sensitivity toward Hg2+ detection make qHCM an attractive probe for detecting Hg2+ in contaminated water sources, which is a major environmental toxicity concern. We have scrutinized the altered metal-ion selectivity of the probe using density functional theory (DFT) and time-dependent DFT calculations, which show that a PET-based metal-sensing scheme is not operational in qHCM. 1H NMR studies and DFT calculations indicate that Hg2+ ions coordinate to oxygen-donor atoms from both the chromophore and macrocycle, leading to sensitive mercury detection.
中文翻译:

用于Hg 2+检测的灵敏水溶性可逆光学探针
我们在尝试开发水溶性锰探针时报告了偶然发现的光学水银传感器。该传感器基于与半菁染料共轭的五氮杂大环。我们小组中较早设计的pentaaza大环用于开发基于光致电子转移(PET)的锰“开启”荧光传感器。(1)为了增加锰传感器的水溶性,我们改变了染料从BODIPY到半菁。生成的分子qHCM在与Hg 2+离子结合后,吸收特征发生了明显的可逆变化,并伴随了可见的颜色变化,从而导致了高度水溶性的汞传感器,其检测极限为10 ppb。该分子可作为汞的可逆“ ON-OFF”荧光传感器2+与35倍的发光强度在1个当量汞的存在降低2+离子。我们已经证明了该探针适用于使用可见光共聚焦荧光显微镜检测活细胞和活斑马鱼幼虫中的Hg 2+离子。对Hg 2+检测的高选择性和灵敏度使qHCM成为用于检测受污染水源中Hg 2+的引人注目的探针,这是一个主要的环境毒性问题。我们已经使用密度泛函理论(DFT)和随时间变化的DFT计算来检查探针改变的金属离子选择性,这表明基于qHCM的基于PET的金属传感方案不起作用。1 H NMR研究和DFT计算表明,Hg 2+离子与发色团和大环上的氧供体原子配位,导致灵敏的汞检测。
更新日期:2018-04-18
中文翻译:

用于Hg 2+检测的灵敏水溶性可逆光学探针
我们在尝试开发水溶性锰探针时报告了偶然发现的光学水银传感器。该传感器基于与半菁染料共轭的五氮杂大环。我们小组中较早设计的pentaaza大环用于开发基于光致电子转移(PET)的锰“开启”荧光传感器。(1)为了增加锰传感器的水溶性,我们改变了染料从BODIPY到半菁。生成的分子qHCM在与Hg 2+离子结合后,吸收特征发生了明显的可逆变化,并伴随了可见的颜色变化,从而导致了高度水溶性的汞传感器,其检测极限为10 ppb。该分子可作为汞的可逆“ ON-OFF”荧光传感器2+与35倍的发光强度在1个当量汞的存在降低2+离子。我们已经证明了该探针适用于使用可见光共聚焦荧光显微镜检测活细胞和活斑马鱼幼虫中的Hg 2+离子。对Hg 2+检测的高选择性和灵敏度使qHCM成为用于检测受污染水源中Hg 2+的引人注目的探针,这是一个主要的环境毒性问题。我们已经使用密度泛函理论(DFT)和随时间变化的DFT计算来检查探针改变的金属离子选择性,这表明基于qHCM的基于PET的金属传感方案不起作用。1 H NMR研究和DFT计算表明,Hg 2+离子与发色团和大环上的氧供体原子配位,导致灵敏的汞检测。































 京公网安备 11010802027423号
京公网安备 11010802027423号