当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-04-26 , DOI: 10.1021/acs.est.8b00735 Yi Yang 1 , Gourab Banerjee , Gary W. Brudvig , Jae-Hong Kim , Joseph J. Pignatello 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-04-26 , DOI: 10.1021/acs.est.8b00735 Yi Yang 1 , Gourab Banerjee , Gary W. Brudvig , Jae-Hong Kim , Joseph J. Pignatello 1
Affiliation
|
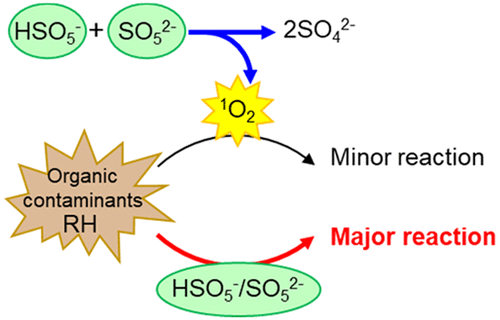
Peroxymonosulfate (HSO5– and PMS) is an optional bulk oxidant in advanced oxidation processes (AOPs) for treating wastewaters. Normally, PMS is activated by the input of energy or reducing agent to generate sulfate or hydroxyl radicals or both. This study shows that PMS without explicit activation undergoes direct reaction with a variety of compounds, including antibiotics, pharmaceuticals, phenolics, and commonly used singlet-oxygen (1O2) traps and quenchers, specifically furfuryl alcohol (FFA), azide, and histidine. Reaction time frames varied from minutes to a few hours at pH 9. With the use of a test compound with intermediate reactivity (FFA), electron paramagnetic resonance (EPR) and scavenging experiments ruled out sulfate and hydroxyl radicals. Although 1O2 was detected by EPR and is produced stoichiometrically through PMS self-decomposition, 1O2 plays only a minor role due to its efficient quenching by water, as confirmed by experiments manipulating the 1O2 formation rate (addition of H2O2) or lifetime (deuterium solvent isotope effect). Direct reactions with PMS are highly pH- and ionic-strength-sensitive and can be accelerated by (bi)carbonate, borate, and pyrophosphate (although not phosphate) via non-radical pathways. The findings indicate that direct reaction with PMS may steer degradation pathways and must be considered in AOPs and other applications. They also signal caution to researchers when choosing buffers as well as 1O2 traps and quenchers.
中文翻译:

未活化的过一硫酸盐氧化水中的有机化合物
过氧单(HSO 5 -和PMS)是在高级氧化处理(高级氧化)一种治疗废水可选散装氧化剂。通常,通过输入能量或还原剂激活PMS,以生成硫酸盐或羟基或两者。这项研究表明,未经明确激活的PMS会与多种化合物直接反应,包括抗生素,药物,酚类和常用的单线态氧(1 O 2)捕集剂和猝灭剂,特别是糠醇(FFA),叠氮化物和组氨酸。在pH值为9的情况下,反应时间范围从数分钟到数小时不等。使用具有中等反应性(FFA)的受试化合物,电子顺磁共振(EPR)和清除实验排除了硫酸根和羟基自由基。尽管通过EPR检测到1 O 2并通过PMS自分解化学计量生成1 O 2,但是如通过操纵1 O 2形成速率(添加H 2的实验)所证实的,1 O 2由于有效地被水淬灭而仅起次要作用。Ø 2)或寿命(氘代溶剂同位素效应)。与PMS的直接反应对pH和离子强度非常敏感,并且可以通过非自由基途径通过(碳酸)碳酸盐,硼酸盐和焦磷酸盐(尽管不是磷酸盐)来加速。研究结果表明,与PMS的直接反应可能会引导降解途径,因此在AOP和其他应用中必须加以考虑。选择缓冲以及当他们还警告信号,研究人员1 Ò 2个陷阱和淬灭剂。
更新日期:2018-04-27
中文翻译:

未活化的过一硫酸盐氧化水中的有机化合物
过氧单(HSO 5 -和PMS)是在高级氧化处理(高级氧化)一种治疗废水可选散装氧化剂。通常,通过输入能量或还原剂激活PMS,以生成硫酸盐或羟基或两者。这项研究表明,未经明确激活的PMS会与多种化合物直接反应,包括抗生素,药物,酚类和常用的单线态氧(1 O 2)捕集剂和猝灭剂,特别是糠醇(FFA),叠氮化物和组氨酸。在pH值为9的情况下,反应时间范围从数分钟到数小时不等。使用具有中等反应性(FFA)的受试化合物,电子顺磁共振(EPR)和清除实验排除了硫酸根和羟基自由基。尽管通过EPR检测到1 O 2并通过PMS自分解化学计量生成1 O 2,但是如通过操纵1 O 2形成速率(添加H 2的实验)所证实的,1 O 2由于有效地被水淬灭而仅起次要作用。Ø 2)或寿命(氘代溶剂同位素效应)。与PMS的直接反应对pH和离子强度非常敏感,并且可以通过非自由基途径通过(碳酸)碳酸盐,硼酸盐和焦磷酸盐(尽管不是磷酸盐)来加速。研究结果表明,与PMS的直接反应可能会引导降解途径,因此在AOP和其他应用中必须加以考虑。选择缓冲以及当他们还警告信号,研究人员1 Ò 2个陷阱和淬灭剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号