当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Difluorocarbene as a Building Block for Consecutive Bond-Forming Reactions
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00079 Alexander D. Dilman 1 , Vitalij V. Levin 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-04-17 00:00:00 , DOI: 10.1021/acs.accounts.8b00079 Alexander D. Dilman 1 , Vitalij V. Levin 1
Affiliation
|
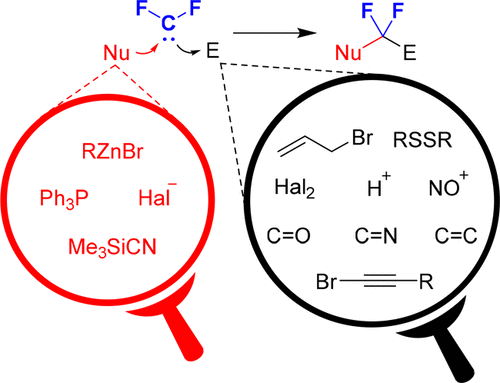
Compounds containing a difluoromethylene unit have gained increasing attention due to their utility in drug design. Classic methods for the synthesis of these compounds rely on either harsh deoxofluorination reactions or laborious functional group manipulation sequences. In 2013, we proposed a method for assembling gem-difluorinated molecules from a difluorocarbene, a nucleophile, and an electrophile. In this process, a difluorocarbene can be considered an equivalent of a bipolar CF2 unit. Performing consecutive bond-forming reactions by sequential attachment of a nucleophile and an electrophile to a difluorocarbene provides the opportunity for the synthesis of a wide variety of organofluorine compounds. Silicon reagents were the most effective sources of the difluoromethylene fragment, and among them (bromodifluoromethyl)trimethylsilane (Me3SiCF2Br) is the reagent of choice. Mildly basic activators such HMPA, DMPU, bromide and acetate ions can initiate the decomposition of the silane with concomitant generation of a difluorocarbene.
中文翻译:

二氟卡宾作为连续键形成反应的基础
含有二氟亚甲基单元的化合物由于其在药物设计中的用途而受到越来越多的关注。合成这些化合物的经典方法依赖于苛刻的脱氧氟化反应或费力的官能团操纵序列。2013年,我们提出了一种从二氟卡宾,亲核试剂和亲电试剂组装宝石二氟化分子的方法。在此过程中,可以将二氟卡宾视为双极性CF 2的等价物。单元。通过将亲核试剂和亲电试剂顺序连接到二氟卡宾上进行连续的键形成反应,为合成多种有机氟化合物提供了机会。硅试剂是二氟亚甲基片段的最有效来源,其中(溴二氟甲基)三甲基硅烷(Me 3 SiCF 2 Br)是首选试剂。温和的碱性活化剂,例如HMPA,DMPU,溴离子和乙酸根离子,可以引发硅烷的分解,并伴随生成二氟卡宾。
更新日期:2018-04-17
中文翻译:

二氟卡宾作为连续键形成反应的基础
含有二氟亚甲基单元的化合物由于其在药物设计中的用途而受到越来越多的关注。合成这些化合物的经典方法依赖于苛刻的脱氧氟化反应或费力的官能团操纵序列。2013年,我们提出了一种从二氟卡宾,亲核试剂和亲电试剂组装宝石二氟化分子的方法。在此过程中,可以将二氟卡宾视为双极性CF 2的等价物。单元。通过将亲核试剂和亲电试剂顺序连接到二氟卡宾上进行连续的键形成反应,为合成多种有机氟化合物提供了机会。硅试剂是二氟亚甲基片段的最有效来源,其中(溴二氟甲基)三甲基硅烷(Me 3 SiCF 2 Br)是首选试剂。温和的碱性活化剂,例如HMPA,DMPU,溴离子和乙酸根离子,可以引发硅烷的分解,并伴随生成二氟卡宾。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号