当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potent Inhibitors of Plasmodial Serine Hydroxymethyltransferase (SHMT) Featuring a Spirocyclic Scaffold
ChemMedChem ( IF 3.6 ) Pub Date : 2018-04-14 , DOI: 10.1002/cmdc.201800053
Geoffrey Schwertz 1 , Matthias C. Witschel 2 , Matthias Rottmann 3, 4 , Ubolsree Leartsakulpanich 5 , Penchit Chitnumsub 5 , Aritsara Jaruwat 5 , Watcharee Amornwatcharapong 6 , Wanwipa Ittarat 5 , Anja Schäfer 3, 4 , Raphael A. Aponte 2 , Nils Trapp 1 , Pimchai Chaiyen 6, 7 , François Diederich 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-04-14 , DOI: 10.1002/cmdc.201800053
Geoffrey Schwertz 1 , Matthias C. Witschel 2 , Matthias Rottmann 3, 4 , Ubolsree Leartsakulpanich 5 , Penchit Chitnumsub 5 , Aritsara Jaruwat 5 , Watcharee Amornwatcharapong 6 , Wanwipa Ittarat 5 , Anja Schäfer 3, 4 , Raphael A. Aponte 2 , Nils Trapp 1 , Pimchai Chaiyen 6, 7 , François Diederich 1
Affiliation
|
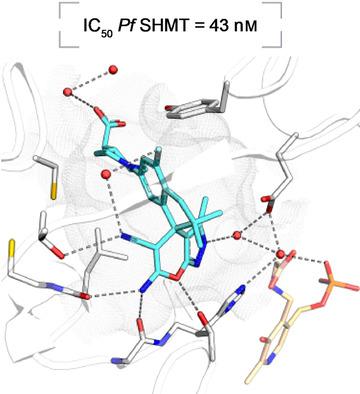
With the discovery that serine hydroxymethyltransferase (SHMT) is a druggable target for antimalarials, the aim of this study was to design novel inhibitors of this key enzyme in the folate biosynthesis cycle. Herein, 19 novel spirocyclic ligands based on either 2‐indolinone or dihydroindene scaffolds and featuring a pyrazolopyran core are reported. Strong target affinities for Plasmodium falciparum (Pf) SHMT (14–76 nm) and cellular potencies in the low nanomolar range (165–334 nm) were measured together with interesting selectivity against human cytosolic SHMT1 (hSHMT1). Four co‐crystal structures with Plasmodium vivax (Pv) SHMT solved at 2.2–2.4 Å resolution revealed the key role of the vinylogous cyanamide for anchoring ligands within the active site. The spirocyclic motif in the molecules enforces the pyrazolopyran core to adopt a substantially more curved conformation than that of previous non‐spirocyclic analogues. Finally, solvation of the spirocyclic lactam ring of the receptor‐bound ligands is discussed.
中文翻译:

具有螺旋环支架的血浆丝氨酸羟甲基转移酶(SHMT)的强效抑制剂。
随着丝氨酸羟甲基转移酶(SHMT)是抗疟药的可治疗靶点的发现,这项研究的目的是在叶酸生物合成周期中设计该关键酶的新型抑制剂。本文报道了19种基于2-吲哚满酮或二氢茚骨架的新颖螺环配体,并具有吡唑并吡喃核心。测量了对恶性疟原虫(Pf)SHMT(14–76 n m)的强目标亲和力和低纳摩尔范围(165–334 n m)的细胞效能,以及对人胞质SHMT1(h SHMT1)的有趣选择性。间日疟原虫的四个共晶体结构(Pv)SHMT在2.2–2.4Å的分辨率下进行了分析,揭示了乙烯基氰胺对于将配体锚定在活性位点中的关键作用。分子中的螺环基序使吡唑并吡喃核心具有比以前的非螺环类似物更大的弯曲构象。最后,讨论了受体结合的配体的螺内酰胺环的溶剂化。
更新日期:2018-04-14
中文翻译:

具有螺旋环支架的血浆丝氨酸羟甲基转移酶(SHMT)的强效抑制剂。
随着丝氨酸羟甲基转移酶(SHMT)是抗疟药的可治疗靶点的发现,这项研究的目的是在叶酸生物合成周期中设计该关键酶的新型抑制剂。本文报道了19种基于2-吲哚满酮或二氢茚骨架的新颖螺环配体,并具有吡唑并吡喃核心。测量了对恶性疟原虫(Pf)SHMT(14–76 n m)的强目标亲和力和低纳摩尔范围(165–334 n m)的细胞效能,以及对人胞质SHMT1(h SHMT1)的有趣选择性。间日疟原虫的四个共晶体结构(Pv)SHMT在2.2–2.4Å的分辨率下进行了分析,揭示了乙烯基氰胺对于将配体锚定在活性位点中的关键作用。分子中的螺环基序使吡唑并吡喃核心具有比以前的非螺环类似物更大的弯曲构象。最后,讨论了受体结合的配体的螺内酰胺环的溶剂化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号