当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NBS/DMSO-mediated synthesis of (2,3-dihydrobenzo[b][1,4]oxathiin-3-yl)methanols from aryloxymethylthiiranes†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-04-13 00:00:00 , DOI: 10.1039/c8nj01117f Jun Dong 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-04-13 00:00:00 , DOI: 10.1039/c8nj01117f Jun Dong 1, 2, 3, 4, 5 , Jiaxi Xu 1, 2, 3, 4, 5
Affiliation
|
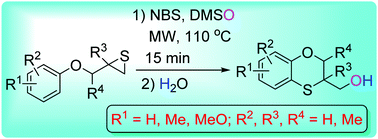
(2,3-Dihydrobenzo[b][1,4]oxathiin-3-yl)methanols were synthesized via reactions of aryloxymethylthiiranes and N-bromosuccinimide (NBS) in DMSO under microwave irradiation. The reaction mechanism was proposed as an intramolecular aromatic electrophilic substitution of 1-bromo-2-(aryloxymethyl)thiiran-1-iums, generated from aryloxymethylthiiranes and NBS, and the subsequent DMSO nucleophilic ring opening reaction of thiiran-1-iums followed by the water displacement. The current method provides a direct and simple strategy in the efficient preparation of (2,3-dihydrobenzo[b][1,4]oxathiin-3-yl)methanols from readily available aryloxymethylthiiranes.
中文翻译:

NBS / DMSO介导的由芳氧基甲基噻喃合成(2,3-二氢苯并[ b ] [1,4]草酰-3-基)甲醇†
通过芳氧基甲基硫烷和N-溴琥珀酰亚胺(NBS)在DMSO下微波辐射反应合成了(2,3-二氢苯并[ b ] [1,4]氧杂嘧啶-3-基)甲醇。该反应机理被认为是由芳氧基甲基噻喃和NBS生成的1-溴-2-(芳氧基甲基)噻喃-1-鎓的分子内芳族亲电取代,以及随后的噻吩-1-鎓的DMSO亲核开环反应,随后是排水量。当前的方法提供了一种直接和简单的策略,可以从容易获得的芳氧基甲基噻喃有效地制备(2,3-二氢苯并[ b ] [1,4]氧杂蒽-3-基)甲醇。
更新日期:2018-04-13
中文翻译:

NBS / DMSO介导的由芳氧基甲基噻喃合成(2,3-二氢苯并[ b ] [1,4]草酰-3-基)甲醇†
通过芳氧基甲基硫烷和N-溴琥珀酰亚胺(NBS)在DMSO下微波辐射反应合成了(2,3-二氢苯并[ b ] [1,4]氧杂嘧啶-3-基)甲醇。该反应机理被认为是由芳氧基甲基噻喃和NBS生成的1-溴-2-(芳氧基甲基)噻喃-1-鎓的分子内芳族亲电取代,以及随后的噻吩-1-鎓的DMSO亲核开环反应,随后是排水量。当前的方法提供了一种直接和简单的策略,可以从容易获得的芳氧基甲基噻喃有效地制备(2,3-二氢苯并[ b ] [1,4]氧杂蒽-3-基)甲醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号