当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conjugation between σ- and π-Aromaticity in 1-C-Arylated Monocarba-closo-dodecaborate Anions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-11-25 , DOI: 10.1021/jacs.5b10321
Mai Otsuka 1 , Ryo Takita 2 , Junichiro Kanazawa 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Masanobu Uchiyama 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2015-11-25 , DOI: 10.1021/jacs.5b10321
Mai Otsuka 1 , Ryo Takita 2 , Junichiro Kanazawa 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Masanobu Uchiyama 1, 2
Affiliation
|
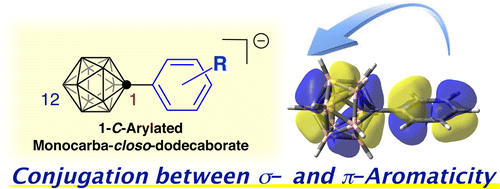
Conjugation between σ- and π-aromatic moieties in 1-C-arylated monocarba-closo-dodecaborate anion derivatives 2 has been identified by means of kinetic experimental studies combined with theoretical calculations. We found that the reaction rate of iodination at the 12-B vertex of the carborane anion cage was affected by distal substituents on the benzene ring connected at the antipodal carbon vertex. Hammett and Yukawa-Tsuno plots indicated that substantial resonance effects are involved. Density functional theory calculations enabled detailed interpretation of the electronic interaction.
中文翻译:

1-C-芳基化单碳-接近-十二硼酸阴离子中σ-和π-芳香性之间的共轭
σ- 和 π- 芳族部分之间的共轭已通过动力学实验研究结合理论计算确定。我们发现碳硼烷阴离子笼的 12-B 顶点处的碘化反应速率受连接在对映碳顶点处的苯环上的远端取代基的影响。Hammett 和 Yukawa-Tsuno 图表明涉及大量共振效应。密度泛函理论计算能够详细解释电子相互作用。
更新日期:2015-11-25
中文翻译:

1-C-芳基化单碳-接近-十二硼酸阴离子中σ-和π-芳香性之间的共轭
σ- 和 π- 芳族部分之间的共轭已通过动力学实验研究结合理论计算确定。我们发现碳硼烷阴离子笼的 12-B 顶点处的碘化反应速率受连接在对映碳顶点处的苯环上的远端取代基的影响。Hammett 和 Yukawa-Tsuno 图表明涉及大量共振效应。密度泛函理论计算能够详细解释电子相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号