Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface-Mediated Protein Unfolding as a Search Process for Denaturing Sites
ACS Nano ( IF 15.8 ) Pub Date : 2015-11-25 00:00:00 , DOI: 10.1021/acsnano.5b05787 James S. Weltz 1 , Daniel K. Schwartz 1 , Joel L. Kaar 1
ACS Nano ( IF 15.8 ) Pub Date : 2015-11-25 00:00:00 , DOI: 10.1021/acsnano.5b05787 James S. Weltz 1 , Daniel K. Schwartz 1 , Joel L. Kaar 1
Affiliation
|
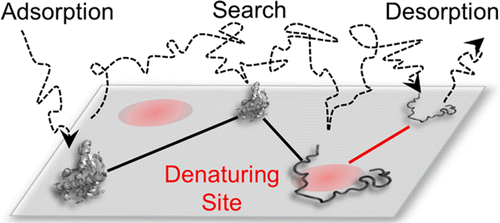
Surface-induced protein denaturation has important implications for the development of materials that are resistant and/or innocuous to biomolecules. Here, we studied the mechanism of lysozyme (T4L) unfolding on fused silica (FS) using single-molecule methods that provided direct insight into the cause of denaturation. Unfolding of T4L was monitored by Förster resonance energy transfer while simultaneously tracking the adsorption, diffusion, and desorption of individual molecules at the solid–solution interface. Results of high-throughput single-molecule analysis suggested that the unfolding of T4L on FS was mediated by surface diffusion and occurred on isolated nanoscale sites, which were relatively rare and distinct from the majority of the surface. These observations suggest that surface-mediated protein unfolding is a search process that is based on the exploration for denaturing sites by the protein. Ultimately, these findings have important implications for the design of protein-compatible surfaces.
中文翻译:

表面介导的蛋白质展开作为变性位点的搜索过程
表面诱导的蛋白质变性对于开发对生物分子具有抗性和/或无害的材料具有重要意义。在这里,我们使用单分子方法研究了溶菌酶(T4L)在熔融石英(FS)上展开的机理,该方法可直接了解变性的原因。通过Förster共振能量转移来监测T4L的展开,同时跟踪固溶体界面上单个分子的吸附,扩散和解吸。高通量单分子分析的结果表明,T4L在FS上的展开是由表面扩散介导的,并发生在孤立的纳米级位点上,这些位点相对稀少且与大多数表面不同。这些观察结果表明,表面介导的蛋白质解折叠是基于蛋白质对变性位点的探索的搜索过程。最终,这些发现对蛋白质兼容表面的设计具有重要意义。
更新日期:2015-11-25
中文翻译:

表面介导的蛋白质展开作为变性位点的搜索过程
表面诱导的蛋白质变性对于开发对生物分子具有抗性和/或无害的材料具有重要意义。在这里,我们使用单分子方法研究了溶菌酶(T4L)在熔融石英(FS)上展开的机理,该方法可直接了解变性的原因。通过Förster共振能量转移来监测T4L的展开,同时跟踪固溶体界面上单个分子的吸附,扩散和解吸。高通量单分子分析的结果表明,T4L在FS上的展开是由表面扩散介导的,并发生在孤立的纳米级位点上,这些位点相对稀少且与大多数表面不同。这些观察结果表明,表面介导的蛋白质解折叠是基于蛋白质对变性位点的探索的搜索过程。最终,这些发现对蛋白质兼容表面的设计具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号