Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of a Novel C11-Acetone Adduct of a Pyrrolobenzodiazepine (PBD) with Loss of Cytotoxicity
Synlett ( IF 1.7 ) Pub Date : 2018-04-10 , DOI: 10.1055/s-0036-1591552 David Thurston 1, 2 , David Corcoran 1, 2 , Pietro Picconi 1 , Connie Chui 1 , George Procopiou 2 , Ilona Pysz 1, 2 , Khondaker Rahman 1, 2
Synlett ( IF 1.7 ) Pub Date : 2018-04-10 , DOI: 10.1055/s-0036-1591552 David Thurston 1, 2 , David Corcoran 1, 2 , Pietro Picconi 1 , Connie Chui 1 , George Procopiou 2 , Ilona Pysz 1, 2 , Khondaker Rahman 1, 2
Affiliation
|
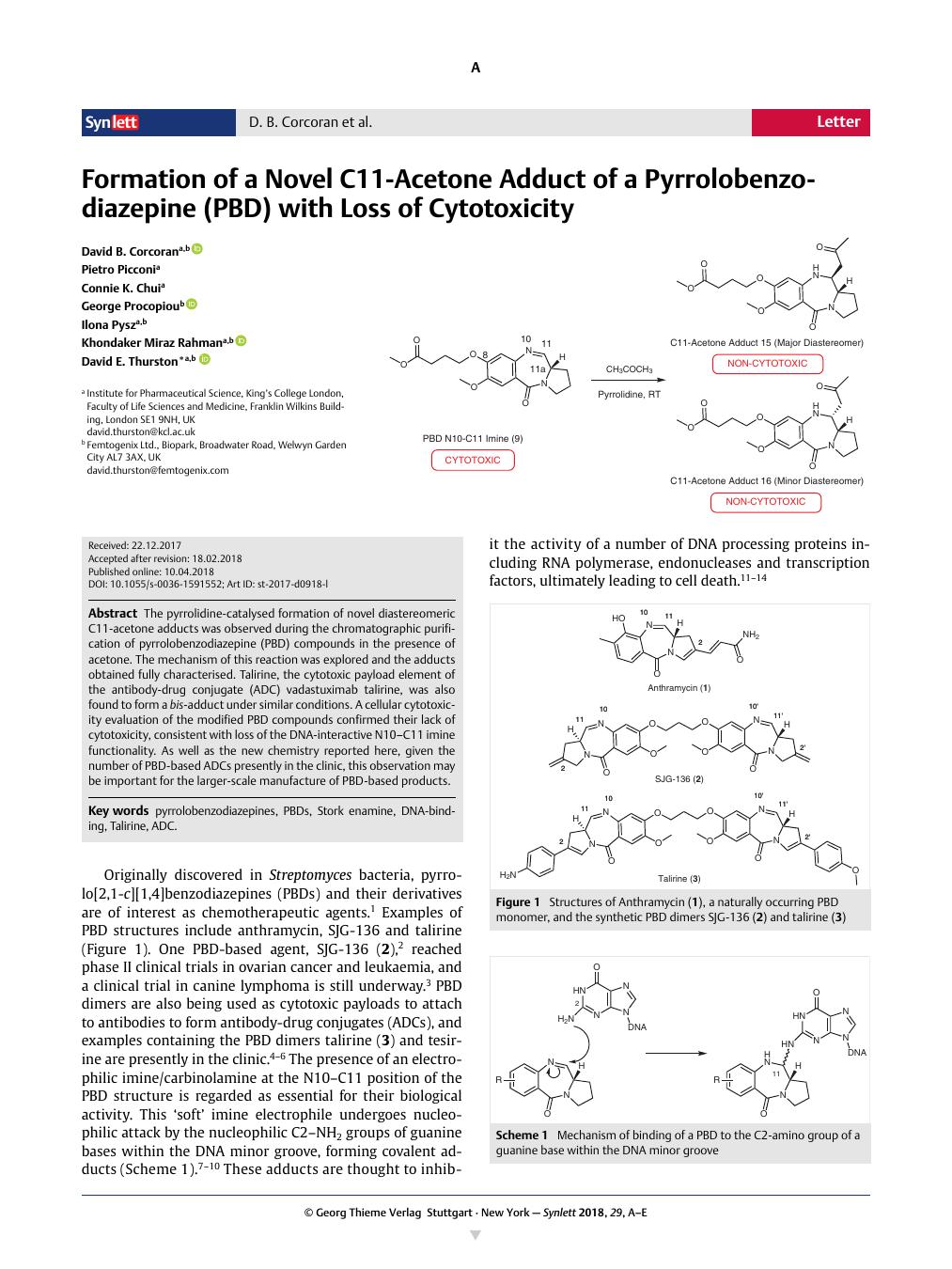
The pyrrolidine-catalysed formation of novel diastereomeric C11-acetone adducts was observed during the chromatographic purification of pyrrolobenzodiazepine (PBD) compounds in the presence of acetone. The mechanism of this reaction was explored and the adducts obtained fully characterised. Talirine, the cytotoxic payload element of the antibody-drug conjugate (ADC) vadastuximab talirine, was also found to form a bis-adduct under similar conditions. A cellular cytotoxicity evaluation of the modified PBD compounds confirmed their lack of cytotoxicity, consistent with loss of the DNA-interactive N10–C11 imine functionality. As well as the new chemistry reported here, given the number of PBD-based ADCs presently in the clinic, this observation may be important for the larger-scale manufacture of PBD-based products.
中文翻译:

失去细胞毒性的吡咯并苯二氮卓 (PBD) 的新型 C11-丙酮加合物的形成
在丙酮存在下,吡咯并苯二氮卓 (PBD) 化合物的色谱纯化过程中观察到了吡咯烷催化形成的新型非对映体 C11-丙酮加合物。探索了该反应的机理并充分表征了所获得的加合物。还发现 Talirine 是抗体-药物偶联物 (ADC) 伐达妥昔单抗 talirine 的细胞毒性有效载荷元素,在类似条件下也可形成双加合物。对修饰的 PBD 化合物的细胞毒性评估证实它们没有细胞毒性,这与 DNA 相互作用的 N10-C11 亚胺功能的丧失一致。除了这里报告的新化学,鉴于目前临床上基于 PBD 的 ADC 的数量,这一观察结果对于大规模生产基于 PBD 的产品可能很重要。
更新日期:2018-04-10
中文翻译:

失去细胞毒性的吡咯并苯二氮卓 (PBD) 的新型 C11-丙酮加合物的形成
在丙酮存在下,吡咯并苯二氮卓 (PBD) 化合物的色谱纯化过程中观察到了吡咯烷催化形成的新型非对映体 C11-丙酮加合物。探索了该反应的机理并充分表征了所获得的加合物。还发现 Talirine 是抗体-药物偶联物 (ADC) 伐达妥昔单抗 talirine 的细胞毒性有效载荷元素,在类似条件下也可形成双加合物。对修饰的 PBD 化合物的细胞毒性评估证实它们没有细胞毒性,这与 DNA 相互作用的 N10-C11 亚胺功能的丧失一致。除了这里报告的新化学,鉴于目前临床上基于 PBD 的 ADC 的数量,这一观察结果对于大规模生产基于 PBD 的产品可能很重要。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号