当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Desymmetrization of meso Tricyclic Systems Derived from Benzene Oxide
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.joc.8b00523 Desirée M. Matías 1 , Jeffrey S. Johnson 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1021/acs.joc.8b00523 Desirée M. Matías 1 , Jeffrey S. Johnson 1
Affiliation
|
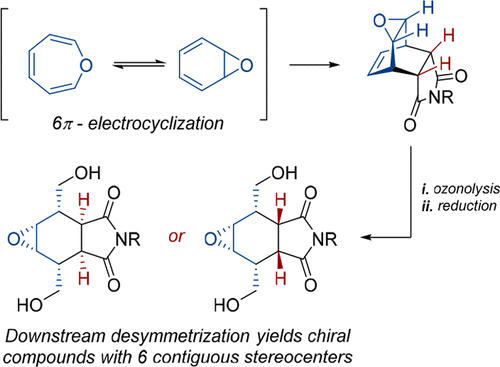
Ozonolysis of the Diels–Alder adducts derived from benzene oxides and N-alkylmaleimides resulted in fully substituted, meso bicyclic systems bearing six contiguous stereocenters, isolated as diols upon reductive workup with NaBH4. Variation in the workup allowed for isolation of two different diastereoisomers, through double epimerization of the imide stereocenters. Desymmetrization of the resulting meso diols via asymmetric nucleophilic epoxide opening and acylation reactions provided access to highly substituted, enantioenriched fused rings.
中文翻译:

氧化苯衍生的内消旋三环体系的合成和不对称化
源自苯氧化物和N-烷基马来酰亚胺的Diels-Alder加合物的臭氧分解导致带有六个连续立体中心的完全取代的内消旋双环体系,经NaBH 4还原后分离为二醇。后处理的变化允许通过酰亚胺立体中心的双差向异构化来分离两种不同的非对映异构体。通过不对称的亲核环氧化物开环和酰化反应使所得的内消旋二醇不对称化,提供了进入高度取代的,对映体富集的稠合环的途径。
更新日期:2018-04-10
中文翻译:

氧化苯衍生的内消旋三环体系的合成和不对称化
源自苯氧化物和N-烷基马来酰亚胺的Diels-Alder加合物的臭氧分解导致带有六个连续立体中心的完全取代的内消旋双环体系,经NaBH 4还原后分离为二醇。后处理的变化允许通过酰亚胺立体中心的双差向异构化来分离两种不同的非对映异构体。通过不对称的亲核环氧化物开环和酰化反应使所得的内消旋二醇不对称化,提供了进入高度取代的,对映体富集的稠合环的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号