当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy lithium-ion batteries†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8ee00155c
Junhyeok Kim 1, 2, 3, 4, 5 , Hyunsoo Ma 1, 2, 3, 4, 5 , Hyungyeon Cha 1, 2, 3, 4, 5 , Hyomyung Lee 1, 2, 3, 4, 5 , Jaekyung Sung 1, 2, 3, 4, 5 , Minho Seo 1, 2, 3, 4, 5 , Pilgun Oh 1, 2, 3, 4, 5 , Minjoon Park 1, 2, 3, 4, 5 , Jaephil Cho 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-04-10 00:00:00 , DOI: 10.1039/c8ee00155c
Junhyeok Kim 1, 2, 3, 4, 5 , Hyunsoo Ma 1, 2, 3, 4, 5 , Hyungyeon Cha 1, 2, 3, 4, 5 , Hyomyung Lee 1, 2, 3, 4, 5 , Jaekyung Sung 1, 2, 3, 4, 5 , Minho Seo 1, 2, 3, 4, 5 , Pilgun Oh 1, 2, 3, 4, 5 , Minjoon Park 1, 2, 3, 4, 5 , Jaephil Cho 1, 2, 3, 4, 5
Affiliation
|
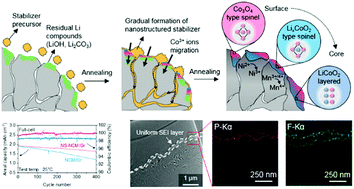
Advanced surface engineering of nickel-rich cathode materials greatly enhances their structural/thermal stability. However, their application into lithium-ion full-cells still faces challenges, such as the unstable solid electrolyte interphase (SEI) layer on the anode. Herein, we reveal that the degradation of battery cycle life is caused by the release of divalent nickel ions from the LiNi0.8Co0.1Mn0.1O2 cathode and the formation of nickel metal particles on the graphite anode surface, deteriorating the anode SEI layer and its structural integrity. On the basis of this finding, we demonstrate a stable lithium-ion battery by modifying the cathode surface by creating a nanostructured stabilizer with an epitaxial structure that enhances the morphological robustness. During cycling, the nickel defects in the cathode are significantly suppressed, preventing nickel ion crossover. In particular, the anode SEI layer maintains a uniform and dense structure, leading to outstanding cycling stability in the full-cell with a capacity retention of ∼86% after 400 cycles at 25 °C.
中文翻译:

通过纳米级外延控制,用于高能锂离子电池的高度稳定的富镍正极材料†
富镍阴极材料的先进表面工程技术大大提高了它们的结构/热稳定性。然而,它们在锂离子全电池中的应用仍然面临挑战,例如阳极上不稳定的固体电解质中间相(SEI)层。在这里,我们揭示了电池循环寿命的降低是由于LiNi 0.8 Co 0.1 Mn 0.1 O 2释放出二价镍离子引起的。阴极和在石墨阳极表面上形成镍金属颗粒,使阳极SEI层及其结构完整性恶化。基于此发现,我们通过创建具有外延结构的纳米结构稳定剂来修饰阴极表面,从而证明了其稳定的锂离子电池性能,该外延结构增强了形态学的鲁棒性。在循环过程中,阴极中的镍缺陷得到了显着抑制,从而防止了镍离子交叉。特别是,阳极SEI层保持均匀且致密的结构,从而在全电池中具有出色的循环稳定性,在25°C下经过400次循环后,容量保持率约为86%。
更新日期:2018-04-10
中文翻译:

通过纳米级外延控制,用于高能锂离子电池的高度稳定的富镍正极材料†
富镍阴极材料的先进表面工程技术大大提高了它们的结构/热稳定性。然而,它们在锂离子全电池中的应用仍然面临挑战,例如阳极上不稳定的固体电解质中间相(SEI)层。在这里,我们揭示了电池循环寿命的降低是由于LiNi 0.8 Co 0.1 Mn 0.1 O 2释放出二价镍离子引起的。阴极和在石墨阳极表面上形成镍金属颗粒,使阳极SEI层及其结构完整性恶化。基于此发现,我们通过创建具有外延结构的纳米结构稳定剂来修饰阴极表面,从而证明了其稳定的锂离子电池性能,该外延结构增强了形态学的鲁棒性。在循环过程中,阴极中的镍缺陷得到了显着抑制,从而防止了镍离子交叉。特别是,阳极SEI层保持均匀且致密的结构,从而在全电池中具有出色的循环稳定性,在25°C下经过400次循环后,容量保持率约为86%。































 京公网安备 11010802027423号
京公网安备 11010802027423号