当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bond Covalency and Oxidation State of Actinide Ions Complexed with Therapeutic Chelating Agent 3,4,3-LI(1,2-HOPO)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00345 Morgan P. Kelley 1 , Gauthier J.-P. Deblonde 2 , Jing Su 1 , Corwin H. Booth 2 , Rebecca J. Abergel 2 , Enrique R. Batista 1 , Ping Yang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-04-06 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00345 Morgan P. Kelley 1 , Gauthier J.-P. Deblonde 2 , Jing Su 1 , Corwin H. Booth 2 , Rebecca J. Abergel 2 , Enrique R. Batista 1 , Ping Yang 1
Affiliation
|
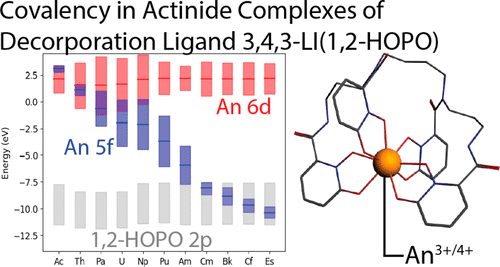
The hydroxypyridinone ligand 3,4,3-LI(1,2-HOPO) is a promising agent for biological decorporation of radionuclides, and allows spectroscopic detection of many lanthanide (Ln) and actinide (An) species via sensitized luminescence. Despite the manifest uses of this ligand, the structural and thermodynamic properties of its complexes across the An series remain understudied. Theoretical investigations of the binding of An(III) and An(IV) ions, from actinium to einsteinium, by the 3,4,3-LI(1,2-HOPO) ligand, as well as experimental extended X-ray absorption fine structure (EXAFS) studies on the trivalent americium, curium, and californium complexes were employed to address the resulting structures, thermodynamic parameters, redox properties, and corresponding electronic configurations. An(IV) ions were found to form much stronger complexes than An(III) ions, consistent with experimental measurements. Complexation of both An(III) and An(IV) ions generally becomes more favorable for heavier actinides, reflecting increased energy degeneracy driven covalency and concomitant orbital mixing between the 5f orbitals of the An ions and the π orbitals of the ligand. Notably, the ability of this ligand to either accept or donate electron density as needed from its pyridine rings is found to be key to its extraordinary stability across the actinide series.
中文翻译:

The离子与治疗性螯合剂3,4,3-LI(1,2-HOPO)的键键合价和氧化态
羟基吡啶酮配体3,4,3-LI(1,2-HOPO)是用于放射性核素生物脱附的有前途的试剂,并允许通过敏化发光光谱法检测许多镧系(Ln)和act系(An)物种。尽管明显使用了该配体,但在整个An系列中其配合物的结构和热力学性质仍未得到充分研究。对3,4,3-LI(1,2-HOPO)配体从act到in的An(III)和An(IV)离子结合的理论研究以及扩展的X射线吸收实验对三价a,cur和配合物进行结构(EXAFS)研究,以解决所得结构,热力学参数,氧化还原特性和相应的电子构型。发现An(IV)离子比An(III)离子形成更强的络合物,与实验测量结果一致。An(III)和An(IV)离子的络合通常对较重的act系元素更有利,这反映了能量简并性驱动的共价性和An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。反映了由能量简并性驱动的共价性增加以及An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。反映了由能量简并性驱动的共价性增加以及An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。
更新日期:2018-04-06
中文翻译:

The离子与治疗性螯合剂3,4,3-LI(1,2-HOPO)的键键合价和氧化态
羟基吡啶酮配体3,4,3-LI(1,2-HOPO)是用于放射性核素生物脱附的有前途的试剂,并允许通过敏化发光光谱法检测许多镧系(Ln)和act系(An)物种。尽管明显使用了该配体,但在整个An系列中其配合物的结构和热力学性质仍未得到充分研究。对3,4,3-LI(1,2-HOPO)配体从act到in的An(III)和An(IV)离子结合的理论研究以及扩展的X射线吸收实验对三价a,cur和配合物进行结构(EXAFS)研究,以解决所得结构,热力学参数,氧化还原特性和相应的电子构型。发现An(IV)离子比An(III)离子形成更强的络合物,与实验测量结果一致。An(III)和An(IV)离子的络合通常对较重的act系元素更有利,这反映了能量简并性驱动的共价性和An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。反映了由能量简并性驱动的共价性增加以及An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。反映了由能量简并性驱动的共价性增加以及An离子的5f轨道与配体的π轨道之间伴随的轨道混合。值得注意的是,发现该配体根据需要从其吡啶环接受或提供电子密度的能力是其在整个act系元素系列中非凡稳定性的关键。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号