Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disease-Causing Mutations in the G Protein Gαs Subvert the Roles of GDP and GTP.
Cell ( IF 45.5 ) Pub Date : 2018-May-17 , DOI: 10.1016/j.cell.2018.03.018 Qi Hu , Kevan M. Shokat
Cell ( IF 45.5 ) Pub Date : 2018-May-17 , DOI: 10.1016/j.cell.2018.03.018 Qi Hu , Kevan M. Shokat
|
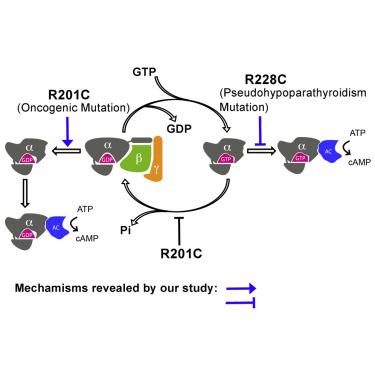
The single most frequent cancer-causing mutation across all heterotrimeric G proteins is R201C in Gαs. The current model explaining the gain-of-function activity of the R201 mutations is through the loss of GTPase activity and resulting inability to switch off to the GDP state. Here, we find that the R201C mutation can bypass the need for GTP binding by directly activating GDP-bound Gαs through stabilization of an intramolecular hydrogen bond network. Having found that a gain-of-function mutation can convert GDP into an activator, we postulated that a reciprocal mutation might disrupt the normal role of GTP. Indeed, we found R228C, a loss-of-function mutation in Gαs that causes pseudohypoparathyroidism type 1a (PHP-Ia), compromised the adenylyl cyclase-activating activity of Gαs bound to a non-hydrolyzable GTP analog. These findings show that disease-causing mutations in Gαs can subvert the canonical roles of GDP and GTP, providing new insights into the regulation mechanism of G proteins.
中文翻译:

G蛋白Gαs中的致病突变破坏了GDP和GTP的作用。
所有异三聚体G蛋白中最常见的致癌突变是Gαs中的R201C。当前解释R201突变的功能获得活性的模型是通过丧失GTPase活性并导致无法转换为GDP状态来进行的。在这里,我们发现R201C突变可通过稳定分子内氢键网络直接激活GDP结合的Gα,从而绕过GTP结合的需要。发现功能增益突变可以将GDP转换为激活因子后,我们推测,相互的突变可能会破坏GTP的正常作用。实际上,我们发现R228C是Gα的功能丧失突变,引起1a型假性甲状旁腺功能减退(PHP-1a),损害了与不可水解的GTP类似物结合的Gα的腺苷酸环化酶激活活性。
更新日期:2018-04-26
中文翻译:

G蛋白Gαs中的致病突变破坏了GDP和GTP的作用。
所有异三聚体G蛋白中最常见的致癌突变是Gαs中的R201C。当前解释R201突变的功能获得活性的模型是通过丧失GTPase活性并导致无法转换为GDP状态来进行的。在这里,我们发现R201C突变可通过稳定分子内氢键网络直接激活GDP结合的Gα,从而绕过GTP结合的需要。发现功能增益突变可以将GDP转换为激活因子后,我们推测,相互的突变可能会破坏GTP的正常作用。实际上,我们发现R228C是Gα的功能丧失突变,引起1a型假性甲状旁腺功能减退(PHP-1a),损害了与不可水解的GTP类似物结合的Gα的腺苷酸环化酶激活活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号