Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Covalent Functionalization of Boron Nitride Nanotubes via Reduction Chemistry
ACS Nano ( IF 15.8 ) Pub Date : 2015-11-23 00:00:00 , DOI: 10.1021/acsnano.5b06523 Homin Shin 1 , Jingwen Guan 1 , Marek Z. Zgierski 1 , Keun Su Kim 1 , Christopher T. Kingston 1 , Benoit Simard 1
ACS Nano ( IF 15.8 ) Pub Date : 2015-11-23 00:00:00 , DOI: 10.1021/acsnano.5b06523 Homin Shin 1 , Jingwen Guan 1 , Marek Z. Zgierski 1 , Keun Su Kim 1 , Christopher T. Kingston 1 , Benoit Simard 1
Affiliation
|
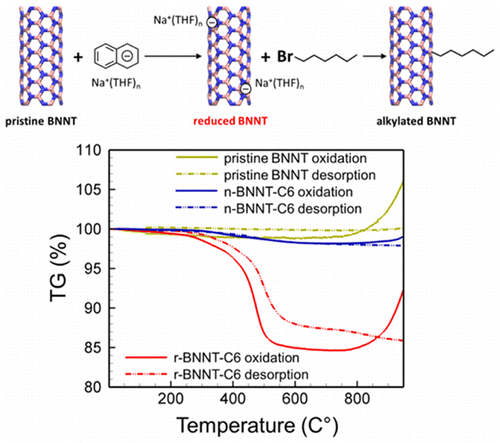
Boron nitride nanotubes (BNNTs) exhibit a range of properties that hold great potential for many fields of science and technology; however, they have inherently low chemical reactivity, making functionalization for specific applications difficult. Here we propose that covalent functionalization of BNNTs via reduction chemistry could be a highly promising and viable strategy. Through density functional theory calculations of the electron affinity of BNNTs and their binding energies with various radicals, we reveal that their chemical reactivity can be significantly enhanced via reducing the nanotubes (i.e., negatively charging). For example, a 5.5-fold enhancement in reactivity of reduced BNNTs toward NH2 radicals was predicted relative to their neutral counterparts. The localization characteristics of the BNNT π electron system lead the excess electrons to fill the empty p orbitals of boron sites, which promote covalent bond formation with an unpaired electron from a radical molecule. In support of our theoretical findings, we also experimentally investigated the covalent alkylation of BNNTs via reduction chemistry using 1-bromohexane. The thermogravimetric measurements showed a considerable weight loss (12–14%) only for samples alkylated using reduced BNNTs, suggesting their significantly improved reactivity over neutral BNNTs. This finding will provide an insight in developing an effective route to chemical functionalization of BNNTs.
中文翻译:

氮化硼纳米管通过还原化学共价官能化
氮化硼纳米管(BNNT)具有一系列特性,在许多科学和技术领域都具有巨大潜力。但是,它们固有的化学反应性较低,因此难以针对特定应用进行功能化。在这里,我们建议通过还原化学对BNNTs进行共价官能化可能是一个非常有前途和可行的策略。通过硼氮纳米管和各种自由基结合能的电子亲合势的密度泛函理论计算,我们揭示了它们的化学活性,可以显著提高通过降低碳纳米管(我。ê,带负电)。例如,还原的BNNT对NH 2的反应性提高了5.5倍相对于中立的相对分子,人们预测过激进分子。BNNTπ电子系统的定位特性导致多余的电子充满硼位的空p轨道,从而促进与自由基分子中未成对电子的共价键形成。为了支持我们的理论发现,我们还使用1-溴己烷通过还原化学方法对BNNT的共价烷基化进行了实验研究。热重法测量表明,仅对于使用还原的BNNT烷基化的样品而言,重量损失显着(12–14%),这表明它们比中性BNNT具有显着改善的反应活性。该发现将为开发有效的BNNTs功能化途径提供见识。
更新日期:2015-11-23
中文翻译:

氮化硼纳米管通过还原化学共价官能化
氮化硼纳米管(BNNT)具有一系列特性,在许多科学和技术领域都具有巨大潜力。但是,它们固有的化学反应性较低,因此难以针对特定应用进行功能化。在这里,我们建议通过还原化学对BNNTs进行共价官能化可能是一个非常有前途和可行的策略。通过硼氮纳米管和各种自由基结合能的电子亲合势的密度泛函理论计算,我们揭示了它们的化学活性,可以显著提高通过降低碳纳米管(我。ê,带负电)。例如,还原的BNNT对NH 2的反应性提高了5.5倍相对于中立的相对分子,人们预测过激进分子。BNNTπ电子系统的定位特性导致多余的电子充满硼位的空p轨道,从而促进与自由基分子中未成对电子的共价键形成。为了支持我们的理论发现,我们还使用1-溴己烷通过还原化学方法对BNNT的共价烷基化进行了实验研究。热重法测量表明,仅对于使用还原的BNNT烷基化的样品而言,重量损失显着(12–14%),这表明它们比中性BNNT具有显着改善的反应活性。该发现将为开发有效的BNNTs功能化途径提供见识。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号