当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Interactions in Zeolite Catalysts and Their Hydrophobically Modified Analogues
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-11-23 00:00:00 , DOI: 10.1021/acscatal.5b02040 Kuizhi Chen 1 , Jarred Kelsey 1 , Jeffery L. White 1 , Lu Zhang 2 , Daniel Resasco 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2015-11-23 00:00:00 , DOI: 10.1021/acscatal.5b02040 Kuizhi Chen 1 , Jarred Kelsey 1 , Jeffery L. White 1 , Lu Zhang 2 , Daniel Resasco 2
Affiliation
|
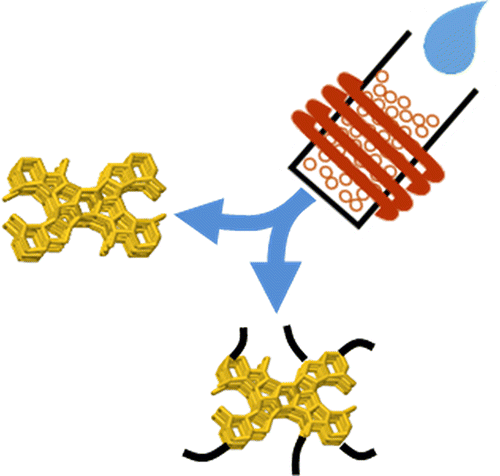
Renewed interest in zeolite catalyst performance in the presence of variable amounts of water has prompted solid-state NMR experiments designed to identify the nature of water interaction with and within conventional and chemically modified H-ZSM-5 zeolites. Recent work has demonstrated that water can positively influence reaction rates in zeolite-catalyzed chemistries, and new interest in catalytic processing of molecules derived from biomass requires understanding the fate of water in and on zeolite catalysts, as a function of water loading. The contribution of acid site density to water adsorption within zeolites is assessed by comparing bulk uptake and molecular experiments at varying Si:Al ratios, and interpreting those results in the context of solid-state NMR results that reveal strongly adsorbed water molecules and water clusters. In situ magic-angle spinning (MAS) NMR experiments for water loadings ranging from ca. 4 to 500 water molecules per zeolite unit cell indicate the following: (1) the dominant interaction is from water adsorbed from the vapor phase at an interior acid site, and unique signals for both the water and acid site are resolved at low loadings; (2) the exchanged-averaged water/acid site chemical shift at higher loadings can be used to measure acid site titration by water; and (3) silane-treated hydrophobically modified H-ZSM-5 does not allow liquid-phase water to access interior acid sites. The in situ1H MAS NMR method indicates that as-synthesized acidic zeolites can be rendered hydrophobic in the presence of liquid-phase water, with only a minimal reduction in the total number of acid sites.
中文翻译:

沸石催化剂中的水相互作用及其疏水改性的类似物
在存在可变量的水的情况下,人们对沸石催化剂性能的新兴趣促使进行了固态NMR实验,旨在确定水与常规和化学改性的H-ZSM-5沸石之间以及与之相互作用的性质。最近的工作表明,水可以对沸石催化的化学反应速率产生积极影响,而对生物质衍生分子的催化处理的新兴趣要求了解沸石催化剂中和沸石催化剂上水的命运与水负荷的关系。通过比较在不同Si:Al比下的大量吸收和分子实验,并在固态NMR结果的背景下解释那些结果,从而揭示出强烈吸附的水分子和水团簇,可以评估酸位点密度对沸石内水吸附的贡献。原位魔角旋转(MAS)NMR实验的水负荷范围约为 每个沸石晶胞有4到500个水分子表示:(1)主要相互作用来自内部酸性部位从气相吸收的水,并且在低负荷下解析出水和酸性部位的独特信号;(2)在较高的负荷下交换的平均水/酸位的化学位移可用于测量水的酸位滴定;(3)硅烷处理的疏水改性H-ZSM-5不允许液相水进入内部酸性位。在原位1H MAS NMR方法表明,在液相水的存在下,所合成的酸性沸石可以变成疏水的,而酸性位点的总数仅有最小的减少。
更新日期:2015-11-23
中文翻译:

沸石催化剂中的水相互作用及其疏水改性的类似物
在存在可变量的水的情况下,人们对沸石催化剂性能的新兴趣促使进行了固态NMR实验,旨在确定水与常规和化学改性的H-ZSM-5沸石之间以及与之相互作用的性质。最近的工作表明,水可以对沸石催化的化学反应速率产生积极影响,而对生物质衍生分子的催化处理的新兴趣要求了解沸石催化剂中和沸石催化剂上水的命运与水负荷的关系。通过比较在不同Si:Al比下的大量吸收和分子实验,并在固态NMR结果的背景下解释那些结果,从而揭示出强烈吸附的水分子和水团簇,可以评估酸位点密度对沸石内水吸附的贡献。原位魔角旋转(MAS)NMR实验的水负荷范围约为 每个沸石晶胞有4到500个水分子表示:(1)主要相互作用来自内部酸性部位从气相吸收的水,并且在低负荷下解析出水和酸性部位的独特信号;(2)在较高的负荷下交换的平均水/酸位的化学位移可用于测量水的酸位滴定;(3)硅烷处理的疏水改性H-ZSM-5不允许液相水进入内部酸性位。在原位1H MAS NMR方法表明,在液相水的存在下,所合成的酸性沸石可以变成疏水的,而酸性位点的总数仅有最小的减少。






























 京公网安备 11010802027423号
京公网安备 11010802027423号