当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Decomposition Mechanism of CL-20 at Different Temperatures by ReaxFF Reactive Molecular Dynamics Simulations
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1021/acs.jpca.8b01256 Fuping Wang 1 , Lang Chen 1 , Deshen Geng 1 , Junying Wu 1 , Jianying Lu 1 , Chen Wang 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-04-05 00:00:00 , DOI: 10.1021/acs.jpca.8b01256 Fuping Wang 1 , Lang Chen 1 , Deshen Geng 1 , Junying Wu 1 , Jianying Lu 1 , Chen Wang 1
Affiliation
|
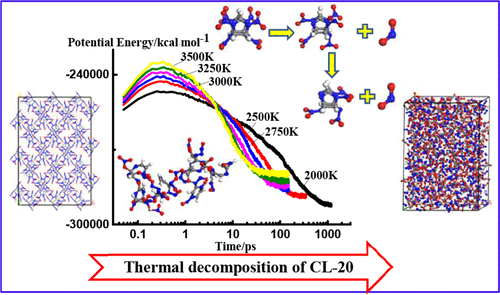
Hexanitrohexaazaisowurtzitane (CL-20) has a high detonation velocity and pressure, but its sensitivity is also high, which somewhat limits its applications. Therefore, it is important to understand the mechanism and characteristics of thermal decomposition of CL-20. In this study, a ε-CL-20 supercell was constructed and ReaxFF-lg reactive molecular dynamics simulations were performed to investigate thermal decomposition of ε-CL-20 at various temperatures (2000, 2500, 2750, 3000, 3250, and 3500 K). The mechanism of thermal decomposition of CL-20 was analyzed from the aspects of potential energy evolution, the primary reactions, and the intermediate and final product species. The effect of temperature on thermal decomposition of CL-20 is also discussed. The initial reaction path of thermal decomposition of CL-20 is N–NO2 cleavage to form NO2, followed by C–N cleavage, leading to the destruction of the cage structure. A small number of clusters appear in the early reactions and disappear at the end of the reactions. The initial reaction path of CL-20 decomposition is the same at different temperatures. However, as the temperature increases, the decomposition rate of CL-20 increases and the cage structure is destroyed earlier. The temperature greatly affects the rate constants of H2O and N2, but it has little effect on the rate constants of CO2 and H2.
中文翻译:

ReaxFF反应分子动力学模拟不同温度下CL-20的热分解机理
六硝基六氮杂异纤锌矿型结构烷烃(CL-20)的爆轰速度和压力较高,但其灵敏度也很高,这在一定程度上限制了其应用。因此,重要的是要了解CL-20的热分解机理和特性。在本研究中,构建了ε-CL-20超级电池,并进行了ReaxFF-lg反应性分子动力学模拟,以研究ε-CL-20在不同温度(2000、2500、2750、3000、3250和3500 K下)的热分解。 )。从势能演化,主要反应以及中间产物和最终产物的种类等方面分析了CL-20的热分解机理。还讨论了温度对CL-20热分解的影响。CL-20热分解的初始反应路径为N–NO 2裂解形成NO 2,然后进行C–N裂解,导致笼结构破坏。少数簇出现在早期反应中,并在反应结束时消失。CL-20分解的初始反应路径在不同温度下是相同的。然而,随着温度升高,CL-20的分解速率增加并且笼状结构更早被破坏。温度极大地影响了H 2 O和N 2的速率常数,但对CO 2和H 2的速率常数影响很小。
更新日期:2018-04-05
中文翻译:

ReaxFF反应分子动力学模拟不同温度下CL-20的热分解机理
六硝基六氮杂异纤锌矿型结构烷烃(CL-20)的爆轰速度和压力较高,但其灵敏度也很高,这在一定程度上限制了其应用。因此,重要的是要了解CL-20的热分解机理和特性。在本研究中,构建了ε-CL-20超级电池,并进行了ReaxFF-lg反应性分子动力学模拟,以研究ε-CL-20在不同温度(2000、2500、2750、3000、3250和3500 K下)的热分解。 )。从势能演化,主要反应以及中间产物和最终产物的种类等方面分析了CL-20的热分解机理。还讨论了温度对CL-20热分解的影响。CL-20热分解的初始反应路径为N–NO 2裂解形成NO 2,然后进行C–N裂解,导致笼结构破坏。少数簇出现在早期反应中,并在反应结束时消失。CL-20分解的初始反应路径在不同温度下是相同的。然而,随着温度升高,CL-20的分解速率增加并且笼状结构更早被破坏。温度极大地影响了H 2 O和N 2的速率常数,但对CO 2和H 2的速率常数影响很小。































 京公网安备 11010802027423号
京公网安备 11010802027423号