当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total synthesis of (±)-tanshinol B, tanshinone I, and (±)-tanshindiol B and C†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8ob00567b Fan Wang 1, 2, 3, 4, 5 , Hong Yang 4, 6, 7, 8, 9 , Shujuan Yu 1, 2, 3, 4, 5 , Yu Xue 5, 7, 8, 9, 10 , Zhoulong Fan 5, 7, 8, 9, 10 , Gaolin Liang 1, 2, 3, 4 , Meiyu Geng 4, 6, 7, 8, 9 , Ao Zhang 5, 7, 8, 9, 10 , Chunyong Ding 5, 7, 8, 9, 10
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-04-04 00:00:00 , DOI: 10.1039/c8ob00567b Fan Wang 1, 2, 3, 4, 5 , Hong Yang 4, 6, 7, 8, 9 , Shujuan Yu 1, 2, 3, 4, 5 , Yu Xue 5, 7, 8, 9, 10 , Zhoulong Fan 5, 7, 8, 9, 10 , Gaolin Liang 1, 2, 3, 4 , Meiyu Geng 4, 6, 7, 8, 9 , Ao Zhang 5, 7, 8, 9, 10 , Chunyong Ding 5, 7, 8, 9, 10
Affiliation
|
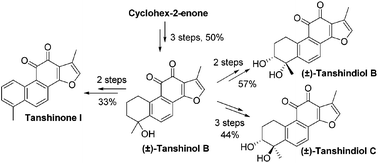
A concise and efficient approach was established for the divergent total synthesis of (±)-tanshindiol B and C and tanshinone I from a ubiquitous ene intermediate in 1–2 steps. This critical intermediate was derived from (±)-tanshinol B, which was synthesized in 50% overall yield over 3 steps using an ultrasound-promoted cycloaddition as a key step. Compared to a previously reported strategy, our approach is more step-economic, thus greatly improving the synthetic efficiency. The bioactivity evaluation indicates that the diol stereochemistry of the tanshidiols has an impact on the EZH2 inhibitory activity.
中文翻译:

(±)-丹参醇B,丹参酮I和(±)-丹参二醇B和C的总合成†
建立了一种简捷有效的方法,以1至2个步骤从无处不在的烯中间体分步进行总合成(±)-丹参二醇B和C和丹参酮I。该关键中间体衍生自(±)-丹参酚B,使用超声波促进的环加成反应为关键步骤,可在3个步骤中以50%的总收率合成。与以前报告的策略相比,我们的方法更具阶梯性,从而大大提高了合成效率。生物活性评估表明,丹参二醇的二醇立体化学对EZH2抑制活性有影响。
更新日期:2018-04-04
中文翻译:

(±)-丹参醇B,丹参酮I和(±)-丹参二醇B和C的总合成†
建立了一种简捷有效的方法,以1至2个步骤从无处不在的烯中间体分步进行总合成(±)-丹参二醇B和C和丹参酮I。该关键中间体衍生自(±)-丹参酚B,使用超声波促进的环加成反应为关键步骤,可在3个步骤中以50%的总收率合成。与以前报告的策略相比,我们的方法更具阶梯性,从而大大提高了合成效率。生物活性评估表明,丹参二醇的二醇立体化学对EZH2抑制活性有影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号