Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling aminophosphine redox mechanisms for glovebox-free InP quantum dot syntheses†
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1039/c8nr01286e Geoffry Laufersky 1, 2, 3, 4, 5 , Siobhan Bradley 1, 2, 3, 4, 5 , Elian Frécaut 3, 4, 5, 6, 7 , Matthias Lein 1, 2, 3, 8, 9 , Thomas Nann 1, 2, 3, 4, 5
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-03 00:00:00 , DOI: 10.1039/c8nr01286e Geoffry Laufersky 1, 2, 3, 4, 5 , Siobhan Bradley 1, 2, 3, 4, 5 , Elian Frécaut 3, 4, 5, 6, 7 , Matthias Lein 1, 2, 3, 8, 9 , Thomas Nann 1, 2, 3, 4, 5
Affiliation
|
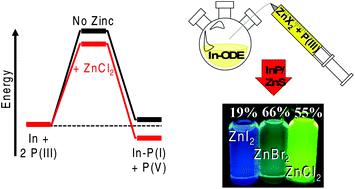
The synthesis of colloidal indium phosphide quantum dots (InP QDs) has always been plagued by difficulties arising from limited P3− sources. Being effectively restricted to the highly pyrophoric tris(trimethylsilyl) phosphine (TMS3P) creates complications for the average chemist and presents a significant risk for industrially scaled reactions. The adaptation of tris(dialkylamino) phosphines for these syntheses has garnered attention, as these new phosphines are much safer and can generate nanoparticles with competitive photoluminescence properties to those from (TMS)3P routes. Until now, the reaction mechanics of this precursor were elusive due to many experimental optimizations, such as the inclusion of a high concentration of zinc salts, being atypical of previous InP syntheses. Herein, we utilize density functional theory calculations to outline a logical reaction mechanism. The aminophosphine precursor is found to require activation by a zinc halide before undergoing a disproportionation reaction to self-reduce this P(III) material to a P(-III) source. We use this understanding to adapt this precursor for a two-pot nanoparticle synthesis in a noncoordinating solvent outside of glovebox conditions. This allowed us to generate spherical InP/ZnS nanoparticles possessing fluorescence quantum yields >55% and lifetimes as fast as 48 ns, with tunable emission according to varying zinc halide acidity. The development of high quality and efficient InP QDs with this safer aminophosphine in simple Schlenk environments will enable a broader range of researchers to synthesize these nontoxic materials for a variety of high-value applications.
中文翻译:

揭开氨基膦氧化还原机理,实现无手套箱的InP量子点合成†
胶态磷化铟量子点(InP QDs)的合成一直受到有限的P 3-来源引起的困难的困扰。有效地限制于高度自燃的三(三甲基甲硅烷基)膦(TMS 3 P)会给普通化学家带来麻烦,并给工业规模的反应带来重大风险。三(二烷基氨基)膦在这些合成中的适应性已引起关注,因为这些新的膦更安全,并且可以产生具有与(TMS)3相比具有竞争性光致发光性能的纳米粒子P条路线。到目前为止,由于许多实验优化(例如,包含高浓度的锌盐,这是以前的InP合成的非典型方法),因此该前体的反应机理仍然难以捉摸。在本文中,我们利用密度泛函理论计算来概述逻辑反应机理。氨基膦前体发现需要通过卤化锌激活经历歧化反应,以在此之前P(自减少III)材料至P( - III) 资源。我们利用这种理解使这种前体适合在手套箱条件之外的非配位溶剂中进行两锅纳米颗粒合成。这使我们能够生成球形InP / ZnS纳米粒子,该纳米粒子的荧光量子产率> 55%,寿命高达48 ns,并且根据卤化锌酸度的变化可调节发射。在简单的Schlenk环境中使用这种更安全的氨基膦开发高质量,高效的InP QD,将使更多的研究人员能够合成这些无毒材料,以用于各种高价值应用。
更新日期:2018-04-03
中文翻译:

揭开氨基膦氧化还原机理,实现无手套箱的InP量子点合成†
胶态磷化铟量子点(InP QDs)的合成一直受到有限的P 3-来源引起的困难的困扰。有效地限制于高度自燃的三(三甲基甲硅烷基)膦(TMS 3 P)会给普通化学家带来麻烦,并给工业规模的反应带来重大风险。三(二烷基氨基)膦在这些合成中的适应性已引起关注,因为这些新的膦更安全,并且可以产生具有与(TMS)3相比具有竞争性光致发光性能的纳米粒子P条路线。到目前为止,由于许多实验优化(例如,包含高浓度的锌盐,这是以前的InP合成的非典型方法),因此该前体的反应机理仍然难以捉摸。在本文中,我们利用密度泛函理论计算来概述逻辑反应机理。氨基膦前体发现需要通过卤化锌激活经历歧化反应,以在此之前P(自减少III)材料至P( - III) 资源。我们利用这种理解使这种前体适合在手套箱条件之外的非配位溶剂中进行两锅纳米颗粒合成。这使我们能够生成球形InP / ZnS纳米粒子,该纳米粒子的荧光量子产率> 55%,寿命高达48 ns,并且根据卤化锌酸度的变化可调节发射。在简单的Schlenk环境中使用这种更安全的氨基膦开发高质量,高效的InP QD,将使更多的研究人员能够合成这些无毒材料,以用于各种高价值应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号