当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for Superoxide Activation of Flavobacterium johnsoniae Class I Ribonucleotide Reductase and for Radical Initiation by Its Dimanganese Cofactor
Biochemistry ( IF 2.9 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00247 Hannah R. Rose , Manas K. Ghosh , Ailiena O. Maggiolo , Christopher J. Pollock , Elizabeth J. Blaesi , Viviane Hajj , Yifeng Wei 1 , Lauren J. Rajakovich , Wei-chen Chang , Yilin Han , Mariana Hajj , Carsten Krebs , Alexey Silakov , Maria-Eirini Pandelia 2 , J. Martin Bollinger , Amie K. Boal
Biochemistry ( IF 2.9 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00247 Hannah R. Rose , Manas K. Ghosh , Ailiena O. Maggiolo , Christopher J. Pollock , Elizabeth J. Blaesi , Viviane Hajj , Yifeng Wei 1 , Lauren J. Rajakovich , Wei-chen Chang , Yilin Han , Mariana Hajj , Carsten Krebs , Alexey Silakov , Maria-Eirini Pandelia 2 , J. Martin Bollinger , Amie K. Boal
Affiliation
|
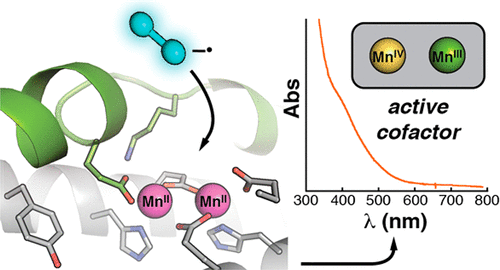
A ribonucleotide reductase (RNR) from Flavobacterium johnsoniae (Fj) differs fundamentally from known (subclass a–c) class I RNRs, warranting its assignment to a new subclass, Id. Its β subunit shares with Ib counterparts the requirements for manganese(II) and superoxide (O2–) for activation, but it does not require the O2–-supplying flavoprotein (NrdI) needed in Ib systems, instead scavenging the oxidant from solution. Although Fj β has tyrosine at the appropriate sequence position (Tyr 104), this residue is not oxidized to a radical upon activation, as occurs in the Ia/b proteins. Rather, Fj β directly deploys an oxidized dimanganese cofactor for radical initiation. In treatment with one-electron reductants, the cofactor can undergo cooperative three-electron reduction to the II/II state, in contrast to the quantitative univalent reduction to inactive “met” (III/III) forms seen with I(a–c) βs. This tendency makes Fj β unusually robust, as the II/II form can readily be reactivated. The structure of the protein rationalizes its distinctive traits. A distortion in a core helix of the ferritin-like architecture renders the active site unusually open, introduces a cavity near the cofactor, and positions a subclass-d-specific Lys residue to shepherd O2– to the Mn2II/II cluster. Relative to the positions of the radical tyrosines in the Ia/b proteins, the unreactive Tyr 104 of Fj β is held away from the cofactor by a hydrogen bond with a subclass-d-specific Thr residue. Structural comparisons, considered with its uniquely simple mode of activation, suggest that the Id protein might most closely resemble the primordial RNR-β.
中文翻译:

约翰逊黄杆菌I类核糖核苷酸还原酶的超氧化物活化及其二锰辅因子自由基引发的结构基础
约翰逊黄杆菌(Fj)的核糖核苷酸还原酶(RNR)与已知的(I–c类)I类RNR根本不同,保证将其分配给新的Id类。其β亚基股磅同行锰(II)和超氧化物(O要求2 - )为激活,但它不需要将O 2 -需要磅系统,而不是清除从溶液中氧化剂-supplying黄素蛋白(NrdI) 。尽管Fjβ在适当的序列位置(Tyr 104)具有酪氨酸,但该残基在激活时不会被氧化成自由基,就像在Ia / b蛋白中那样。而是Fjβ直接将氧化的二锰辅助因子用于自由基引发。在单电子还原剂的处理中,辅因子可以通过三电子协同还原成II / II状态,这与在I(a–c)中看到的定量单价还原成非活性“ met”(III / III)形式相反βs。这种趋势使Fjβ异常坚固,因为II / II形式可以很容易地重新激活。蛋白质的结构使其独特特征合理化。在铁蛋白样结构使活性位点不寻常的开放,介绍了辅因子附近的空腔,和位置的子类-d特异性Lys残基到牧羊犬的O的芯螺旋A失真2 -到的Mn 2 II / II簇。相对于在1a中的自由基酪氨酸的位置/ B的蛋白质,的非反应性的Tyr 104 FJ β由与子类-d特异性Thr残基一个氢键保持从辅因子程。考虑到其独特的简单激活方式,结构比较表明该Id蛋白可能与原始RNR-β最相似。
更新日期:2018-04-02
中文翻译:

约翰逊黄杆菌I类核糖核苷酸还原酶的超氧化物活化及其二锰辅因子自由基引发的结构基础
约翰逊黄杆菌(Fj)的核糖核苷酸还原酶(RNR)与已知的(I–c类)I类RNR根本不同,保证将其分配给新的Id类。其β亚基股磅同行锰(II)和超氧化物(O要求2 - )为激活,但它不需要将O 2 -需要磅系统,而不是清除从溶液中氧化剂-supplying黄素蛋白(NrdI) 。尽管Fjβ在适当的序列位置(Tyr 104)具有酪氨酸,但该残基在激活时不会被氧化成自由基,就像在Ia / b蛋白中那样。而是Fjβ直接将氧化的二锰辅助因子用于自由基引发。在单电子还原剂的处理中,辅因子可以通过三电子协同还原成II / II状态,这与在I(a–c)中看到的定量单价还原成非活性“ met”(III / III)形式相反βs。这种趋势使Fjβ异常坚固,因为II / II形式可以很容易地重新激活。蛋白质的结构使其独特特征合理化。在铁蛋白样结构使活性位点不寻常的开放,介绍了辅因子附近的空腔,和位置的子类-d特异性Lys残基到牧羊犬的O的芯螺旋A失真2 -到的Mn 2 II / II簇。相对于在1a中的自由基酪氨酸的位置/ B的蛋白质,的非反应性的Tyr 104 FJ β由与子类-d特异性Thr残基一个氢键保持从辅因子程。考虑到其独特的简单激活方式,结构比较表明该Id蛋白可能与原始RNR-β最相似。































 京公网安备 11010802027423号
京公网安备 11010802027423号