Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Functional Groups and Interlayer Water Determine the Electrochemical Capacitance of Ti3C2Tx MXene
ACS Nano ( IF 15.8 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acsnano.8b00676 Minmin Hu 1, 2 , Tao Hu 1, 3 , Zhaojin Li 1, 3 , Yi Yang 4 , Renfei Cheng 1, 2 , Jinxing Yang 1, 2 , Cong Cui 1, 2 , Xiaohui Wang 1
ACS Nano ( IF 15.8 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acsnano.8b00676 Minmin Hu 1, 2 , Tao Hu 1, 3 , Zhaojin Li 1, 3 , Yi Yang 4 , Renfei Cheng 1, 2 , Jinxing Yang 1, 2 , Cong Cui 1, 2 , Xiaohui Wang 1
Affiliation
|
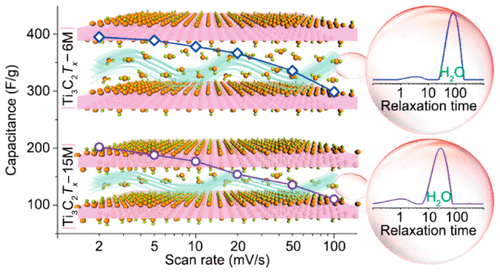
MXenes, an emerging class of conductive two-dimensional materials, have been regarded as promising candidates in the field of electrochemical energy storage. The electrochemical performance of their representative Ti3C2Tx, where T represents the surface termination group of F, O, or OH, strongly relies on termination-mediated surface functionalization, but an in-depth understanding of the relationship between them remains unresolved. Here, we studied comprehensively the structural feature and electrochemical performance of two kinds of Ti3C2Tx MXenes obtained by etching the Ti3AlC2 precursor in aqueous HF solution at low concentration (6 mol/L) and high concentration of (15 mol/L). A significantly higher capacitance was recognized in a low-concentration HF-etched MXene (Ti3C2Tx–6M) electrode. In situ Raman spectroscopy and X-ray photoelectron spectroscopy demonstrate that Ti3C2Tx–6M has more components of the −O functional group. In combination with X-ray diffraction analysis, low-field 1H nuclear magnetic resonance spectroscopy in terms of relaxation time unambiguously underlines that Ti3C2Tx–6M is capable of accommodating more high-mobility H2O molecules between the Ti3C2Tx interlayers, enabling more hydrogen ions to be more readily accessible to the active sites of Ti3C2Tx–6M. The two main key factors (i.e., high content of −O functional groups that are involved bonding/debonding-induced pseudocapacitance and more high-mobility water intercalated between the MXene interlayers) simultaneously account for the superior capacitance of the Ti3C2Tx–6M electrode. This study provides a guideline for the rational design and construction of high-capacitance MXene and MXene-based hybrid electrodes in aqueous electrolytes.
中文翻译:

表面官能团和夹层水决定Ti 3 C 2 T x MXene的电化学电容
MXenes是一类新兴的导电二维材料,在电化学储能领域被认为是很有前途的候选材料。它们的代表Ti 3 C 2 T x的电化学性能(其中T代表F,O或OH的表面端基)在很大程度上取决于端接介导的表面功能化,但尚未深入了解它们之间的关系。 。在这里,我们全面研究了通过蚀刻Ti 3 AlC 2获得的两种Ti 3 C 2 T x MXenes的结构特征和电化学性能。低浓度(6 mol / L)和高浓度(15 mol / L)的HF水溶液中的前驱体。在低浓度的HF蚀刻MXene(Ti 3 C 2 T x –6M)电极中,发现电容明显更高。原位拉曼光谱和X射线光电子能谱表明,Ti 3 C 2 T x –6M具有-O官能团的更多组分。结合X射线衍射分析,就弛豫时间而言,低场1 H核磁共振波谱明确地强调了Ti 3 C 2 T x–6M能够在Ti 3 C 2 T x中间层之间容纳更多的高迁移率H 2 O分子,从而使更多的氢离子更易于进入Ti 3 C 2 T x –6M的活性位。Ti 3 C 2 T x的优异电容是两个主要的关键因素(即,高含量的-O官能团(涉及键/解键合诱导的假电容)和更多的高迁移率水插在MXene中间层之间)–6M电极。这项研究为在水电解质中高容量MXene和基于MXene的混合电极的合理设计和构造提供了指导。
更新日期:2018-04-02
中文翻译:

表面官能团和夹层水决定Ti 3 C 2 T x MXene的电化学电容
MXenes是一类新兴的导电二维材料,在电化学储能领域被认为是很有前途的候选材料。它们的代表Ti 3 C 2 T x的电化学性能(其中T代表F,O或OH的表面端基)在很大程度上取决于端接介导的表面功能化,但尚未深入了解它们之间的关系。 。在这里,我们全面研究了通过蚀刻Ti 3 AlC 2获得的两种Ti 3 C 2 T x MXenes的结构特征和电化学性能。低浓度(6 mol / L)和高浓度(15 mol / L)的HF水溶液中的前驱体。在低浓度的HF蚀刻MXene(Ti 3 C 2 T x –6M)电极中,发现电容明显更高。原位拉曼光谱和X射线光电子能谱表明,Ti 3 C 2 T x –6M具有-O官能团的更多组分。结合X射线衍射分析,就弛豫时间而言,低场1 H核磁共振波谱明确地强调了Ti 3 C 2 T x–6M能够在Ti 3 C 2 T x中间层之间容纳更多的高迁移率H 2 O分子,从而使更多的氢离子更易于进入Ti 3 C 2 T x –6M的活性位。Ti 3 C 2 T x的优异电容是两个主要的关键因素(即,高含量的-O官能团(涉及键/解键合诱导的假电容)和更多的高迁移率水插在MXene中间层之间)–6M电极。这项研究为在水电解质中高容量MXene和基于MXene的混合电极的合理设计和构造提供了指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号