当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of Prephenate Dehydrogenase from the Alternative Tyrosine Biosynthesis Pathway in Plants
ChemBioChem ( IF 2.6 ) Pub Date : 2018-05-02 , DOI: 10.1002/cbic.201800085 Cynthia K Holland 1 , Joseph M Jez 1
ChemBioChem ( IF 2.6 ) Pub Date : 2018-05-02 , DOI: 10.1002/cbic.201800085 Cynthia K Holland 1 , Joseph M Jez 1
Affiliation
|
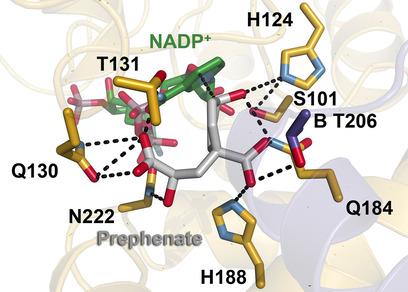
Small changes, big impact: Active site mutants of prephenate dehydrogenase from Glycine max (soybean) were biochemically analyzed by using molecular docking and steady‐state kinetics. The results suggested that even small changes in the active site led to drastic reductions in catalytic efficiency, thus leading us to conclude that nature has optimized this plant tyrosine biosynthetic enzyme.

中文翻译:

植物替代酪氨酸生物合成途径预苯酸脱氢酶的反应机制
小变化,大影响:利用分子对接和稳态动力学对大豆(大豆)预苯酸脱氢酶的活性位点突变体进行生化分析。结果表明,即使活性位点发生微小变化,也会导致催化效率急剧降低,因此我们得出结论,大自然已经优化了这种植物酪氨酸生物合成酶。

更新日期:2018-05-02

中文翻译:

植物替代酪氨酸生物合成途径预苯酸脱氢酶的反应机制
小变化,大影响:利用分子对接和稳态动力学对大豆(大豆)预苯酸脱氢酶的活性位点突变体进行生化分析。结果表明,即使活性位点发生微小变化,也会导致催化效率急剧降低,因此我们得出结论,大自然已经优化了这种植物酪氨酸生物合成酶。



















































 京公网安备 11010802027423号
京公网安备 11010802027423号