当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Water Oxidation Catalyzed by a Mononuclear Iron Complex with a Square Polypyridine Ligand: A DFT Study
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00333 Ying-Ying Li 1 , Lian-Peng Tong 2 , Rong-Zhen Liao 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-03-30 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00333 Ying-Ying Li 1 , Lian-Peng Tong 2 , Rong-Zhen Liao 1
Affiliation
|
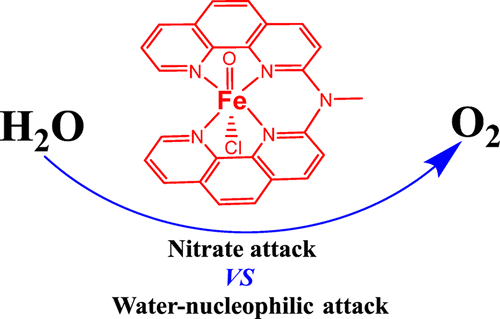
The mononuclear [Cl–FeIII(dpa)–Cl]+ (1Cl) complex containing a square planar tetradentate polypyridine ligand has been reported to catalyze water oxidation in pH = 1 aqueous medium with ceric ammonium nitrate (CAN) as a chemical oxidant. The reaction mechanism of the oxygen evolution driven by this catalyst was investigated by means of density functional calculations. The results showed that one chloride ligand of 1Cl has to exchange with a water molecule to generate 1, [Cl–FeIII(dpa)–OH2]2+, as the starting species of the catalytic cycle. The initial one-electron oxidation of 1 is coupled with the release of two protons, generating [Cl–FeIV(dpa)═O]+ (2). Another one-electron transfer from 2 leads to the formation of an FeV═O complex [Cl–FeV(dpa)═O]2+ (3), which triggers the critical O–O bond formation. The electronic structure of 3 was found to be very similar to that of the high-valent heme-iron center of P450 enzymes, termed Compound I, in which a π-cation radical ligand is believed to support a formal iron(IV)-oxo core. More importantly, 3 and Compound I share the same tendency toward electrophilic reactions. Two competing pathways were suggested for the O–O bond formation based on the present calculations. One is the nitrate nucleophilic attack on the iron(V)-oxo moiety with a total barrier of 12.3 kcal mol–1. In this case, nitrate functions as a co-catalyst for the dioxygen formation. The other is the water nucleophilic attack on iron(V)-oxo with a greater barrier of 16.5 kcal mol–1. In addition, ligand degradation via methyl hydrogen abstraction was found to have a barrier similar to that of the O–O bond formation, while the aromatic carbon hydroxylation has a higher barrier.
中文翻译:

方形多吡啶配体的单核铁配合物催化水氧化的机理:DFT研究
据报道,含有方形平面四齿聚吡啶配体的单核[Cl-Fe III(dpa)-Cl] +(1 Cl)络合物可在pH = 1的水介质中以硝酸铈铵(CAN)作为化学氧化剂催化水氧化。 。通过密度泛函计算研究了由该催化剂驱动的氧气逸出的反应机理。结果表明,一个1 Cl的氯化物配体必须与水分子交换以生成1 [Cl-Fe III(dpa)-OH 2 ] 2+,作为催化循环的起始物种。的初始单电子氧化1结合两个质子的释放,生成[Cl-Fe IV(dpa)═O] +(2)。从另一个电子转移2个导致形成一种Fe的V = O络合物[氯-铁V(DPA)= O] 2+(3),这将触发临界O-O键的形成。发现3的电子结构与称为化合物I的P450酶的高价血红素-铁中心的电子结构非常相似,其中π阳离子自由基配体被认为支持形式铁(IV)-氧代核。更重要的是3化合物I与亲电反应具有相同的趋势。根据目前的计算,提出了O-O键形成的两种竞争途径。一种是硝酸盐对铁(V)-氧代部分的亲核攻击,总势垒为12.3 kcal mol –1。在这种情况下,硝酸盐用作形成双氧的助催化剂。另一个是水对铁(V)-氧的亲核攻击,其阻隔性更大,为16.5 kcal mol –1。此外,发现通过甲基氢提取的配体降解具有与O–O键形成相似的屏障,而芳族碳羟基化具有更高的屏障。
更新日期:2018-03-30
中文翻译:

方形多吡啶配体的单核铁配合物催化水氧化的机理:DFT研究
据报道,含有方形平面四齿聚吡啶配体的单核[Cl-Fe III(dpa)-Cl] +(1 Cl)络合物可在pH = 1的水介质中以硝酸铈铵(CAN)作为化学氧化剂催化水氧化。 。通过密度泛函计算研究了由该催化剂驱动的氧气逸出的反应机理。结果表明,一个1 Cl的氯化物配体必须与水分子交换以生成1 [Cl-Fe III(dpa)-OH 2 ] 2+,作为催化循环的起始物种。的初始单电子氧化1结合两个质子的释放,生成[Cl-Fe IV(dpa)═O] +(2)。从另一个电子转移2个导致形成一种Fe的V = O络合物[氯-铁V(DPA)= O] 2+(3),这将触发临界O-O键的形成。发现3的电子结构与称为化合物I的P450酶的高价血红素-铁中心的电子结构非常相似,其中π阳离子自由基配体被认为支持形式铁(IV)-氧代核。更重要的是3化合物I与亲电反应具有相同的趋势。根据目前的计算,提出了O-O键形成的两种竞争途径。一种是硝酸盐对铁(V)-氧代部分的亲核攻击,总势垒为12.3 kcal mol –1。在这种情况下,硝酸盐用作形成双氧的助催化剂。另一个是水对铁(V)-氧的亲核攻击,其阻隔性更大,为16.5 kcal mol –1。此外,发现通过甲基氢提取的配体降解具有与O–O键形成相似的屏障,而芳族碳羟基化具有更高的屏障。













































 京公网安备 11010802027423号
京公网安备 11010802027423号