Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of the Water Adsorption Properties and Structural Stability of MIL-100(Fe) with Different Anions
Langmuir ( IF 3.7 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b04399
Yen-Ru Chen,Kai-Hsin Liou,Dun-Yen Kang,Jiun-Jen Chen,Li-Chiang Lin
Langmuir ( IF 3.7 ) Pub Date : 2018-03-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b04399
Yen-Ru Chen,Kai-Hsin Liou,Dun-Yen Kang,Jiun-Jen Chen,Li-Chiang Lin
|
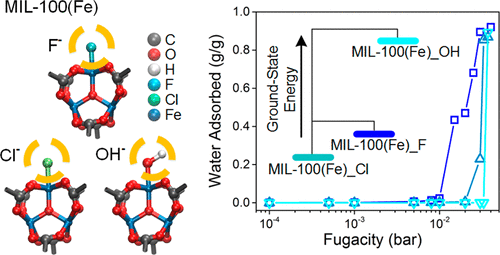
Investigating metal–organic frameworks (MOFs) as water adsorbents has drawn increasing attention for their potential in energy-related applications such as water production and heat transformation. A specific MOF, MIL-100(Fe), is of particular interest for its large adsorption capacity with the occurrence of water condensation at a relatively low partial pressure. In the synthesis of MIL-100(Fe), depending on the reactants, structures with varying anion terminals (e.g., F–, Cl–, or OH–) on the metal trimer have been reported. In this study, we employed molecular simulations and density functional theory calculations for investigating the water adsorption behaviors and the relative structural stability of MIL-100(Fe) with different anions. We also proposed a possible defective structure and explored its water adsorption properties. The results of this study are in good agreement with the experimental measurements and are in support of the observations reported in the literature. Understanding the spatial configurations and energetics of water molecules in these materials has also shed light on their adsorption mechanism at the atomic level.
中文翻译:

不同阴离子的MIL-100(Fe)的吸水性能和结构稳定性的研究
研究金属有机框架(MOF)作为水吸附剂,因其在与能源相关的应用(例如水生产和热转化)中的潜力而引起了越来越多的关注。特定的MOF MIL-100(Fe)因其大的吸附能力以及在相对较低的分压下发生水凝结而特别受关注。在MIL-100(Fe)的合成中,取决于反应物,具有不同阴离子末端的结构(例如F –,Cl –或OH –)在金属三聚体上的报道。在这项研究中,我们采用分子模拟和密度泛函理论计算来研究不同阴离子的MIL-100(Fe)的水吸附行为和相对结构稳定性。我们还提出了一种可能的缺陷结构,并探讨了其吸水性能。这项研究的结果与实验测量结果非常吻合,并支持了文献中报道的观察结果。了解这些材料中水分子的空间构型和能量学也可以阐明它们在原子水平上的吸附机理。
更新日期:2018-03-13
中文翻译:

不同阴离子的MIL-100(Fe)的吸水性能和结构稳定性的研究
研究金属有机框架(MOF)作为水吸附剂,因其在与能源相关的应用(例如水生产和热转化)中的潜力而引起了越来越多的关注。特定的MOF MIL-100(Fe)因其大的吸附能力以及在相对较低的分压下发生水凝结而特别受关注。在MIL-100(Fe)的合成中,取决于反应物,具有不同阴离子末端的结构(例如F –,Cl –或OH –)在金属三聚体上的报道。在这项研究中,我们采用分子模拟和密度泛函理论计算来研究不同阴离子的MIL-100(Fe)的水吸附行为和相对结构稳定性。我们还提出了一种可能的缺陷结构,并探讨了其吸水性能。这项研究的结果与实验测量结果非常吻合,并支持了文献中报道的观察结果。了解这些材料中水分子的空间构型和能量学也可以阐明它们在原子水平上的吸附机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号