当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decomposition Properties of C4F7N/N2 Gas Mixture: An Environmentally Friendly Gas to Replace SF6
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acs.iecr.8b00010
Yi Li 1 , Xiaoxing Zhang 1 , Song Xiao 1 , Qi Chen 1 , Ju Tang 1 , Dachang Chen 1 , Dibo Wang 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-04-02 00:00:00 , DOI: 10.1021/acs.iecr.8b00010
Yi Li 1 , Xiaoxing Zhang 1 , Song Xiao 1 , Qi Chen 1 , Ju Tang 1 , Dachang Chen 1 , Dibo Wang 2
Affiliation
|
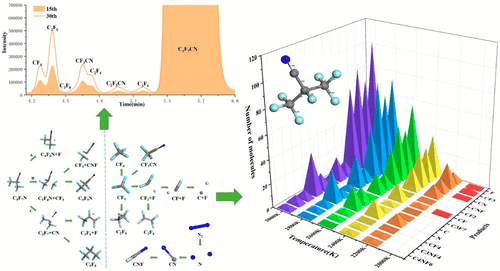
The insulation characteristics and decomposition components of C4F7N/N2 gas mixture, a potential substitute for SF6, were first explored by breakdown experiments/gas chromatography–mass spectrometer. The structural properties of C4F7N molecule and the decomposition mechanism of C4F7N/N2 gas mixture were analyzed based on the density functional theory calculation and ReaxFF molecular dynamics simulation. We found that C4F7N/N2 mixture has great self-recovery performance. The decomposition of C4F7N in a discharge mainly produces CF4, C2F6, C3F8, CF3CN, C2F4, C3F6, and C2F5CN, among which the relative content of C2F6, CF4, and CF3CN is higher. ReaxFF-MD simulations show that CF3, CN, F, and C3F7 are the four main free radicals produced by C4F7N. The decomposition characteristics of N2 are better than that of C4F7N. The addition of N2 has a certain buffering effect to avoid the massive decomposition of C4F7N. The global warming potential value of a gas mixture containing 20% C4F7N decreased by 94.32% compared with SF6. Relevant results not only reveal the decomposition characteristics of C4F7N/N2 mixture in a discharge comprehensively, but also provide a reference for engineering application and emission of a C4F7N/N2 gas mixture.
中文翻译:

C 4 F 7 N / N 2气体混合物的分解特性:一种替代SF 6的环保气体
首先通过击穿实验/气相色谱-质谱仪研究了可能替代SF 6的C 4 F 7 N / N 2气体混合物的绝缘特性和分解成分。基于密度泛函理论计算和ReaxFF分子动力学模拟,分析了C 4 F 7 N分子的结构特性和C 4 F 7 N / N 2气体混合物的分解机理。我们发现,C 4 F 7 N / N 2混合物具有良好的自恢复性能。C 4 F的分解放电中的7 N主要产生CF 4,C 2 F 6,C 3 F 8,CF 3 CN,C 2 F 4,C 3 F 6和C 2 F 5 CN,其中C 2 F的相对含量如图6所示,CF 4和CF 3 CN更高。ReaxFF-MD模拟表明,CF 3,CN,F和C 3 F 7是C 4 F 7产生的四个主要自由基N. N的分解特性2是比的C更好4 ˚F 7 N.加入N 2具有一定的缓冲作用,以避免C的大量分解4 ˚F 7 N的气体混合物的全球变暖潜能值含有20%C 4 F 7 N的元素比SF 6减少了94.32%。相关的结果不仅揭示了C的分解特性4 ˚F 7 N / N 2混合物在放电全面,而且还提供了一个C的工程应用和发射一个参考4 ˚F 7 N / N2混合气。
更新日期:2018-04-03
中文翻译:

C 4 F 7 N / N 2气体混合物的分解特性:一种替代SF 6的环保气体
首先通过击穿实验/气相色谱-质谱仪研究了可能替代SF 6的C 4 F 7 N / N 2气体混合物的绝缘特性和分解成分。基于密度泛函理论计算和ReaxFF分子动力学模拟,分析了C 4 F 7 N分子的结构特性和C 4 F 7 N / N 2气体混合物的分解机理。我们发现,C 4 F 7 N / N 2混合物具有良好的自恢复性能。C 4 F的分解放电中的7 N主要产生CF 4,C 2 F 6,C 3 F 8,CF 3 CN,C 2 F 4,C 3 F 6和C 2 F 5 CN,其中C 2 F的相对含量如图6所示,CF 4和CF 3 CN更高。ReaxFF-MD模拟表明,CF 3,CN,F和C 3 F 7是C 4 F 7产生的四个主要自由基N. N的分解特性2是比的C更好4 ˚F 7 N.加入N 2具有一定的缓冲作用,以避免C的大量分解4 ˚F 7 N的气体混合物的全球变暖潜能值含有20%C 4 F 7 N的元素比SF 6减少了94.32%。相关的结果不仅揭示了C的分解特性4 ˚F 7 N / N 2混合物在放电全面,而且还提供了一个C的工程应用和发射一个参考4 ˚F 7 N / N2混合气。

































 京公网安备 11010802027423号
京公网安备 11010802027423号