当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Multicomponent Condensation Reactions of Phenols, Aldehydes, and Boronates Catalyzed by Chiral Biphenols
Organic Letters ( IF 4.9 ) Pub Date : 2015-11-18 00:00:00 , DOI: 10.1021/acs.orglett.5b02954 Keith S Barbato 1 , Yi Luan 1 , Daniele Ramella 1 , James S Panek 1 , Scott E Schaus 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-11-18 00:00:00 , DOI: 10.1021/acs.orglett.5b02954 Keith S Barbato 1 , Yi Luan 1 , Daniele Ramella 1 , James S Panek 1 , Scott E Schaus 1
Affiliation
|
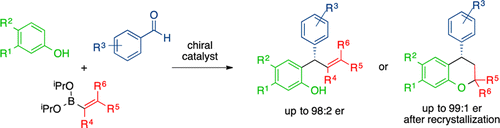
Chiral diols and biphenols catalyze the multicomponent condensation reaction of phenols, aldehydes, and alkenyl or aryl boronates. The condensation products are formed in good yields and enantioselectivities. The reaction proceeds via an initial Friedel–Crafts alkylation of the aldehyde and phenol to yield an ortho-quinone methide that undergoes an enantioselective boronate addition. A cyclization pathway was discovered while exploring the scope of the reaction that provides access to chiral 2,4-diaryl chroman products, the core of which is a structural motif found in natural products.
中文翻译:

手性联苯酚催化的酚、醛和硼酸酯的对映选择性多组分缩合反应
手性二醇和联苯酚催化酚、醛和烯基或芳基硼酸酯的多组分缩合反应。缩合产物以良好的产率和对映选择性形成。该反应通过醛和苯酚的初始弗里德尔-克来福特烷基化进行,产生邻醌甲基化物,然后进行对映选择性硼酸酯加成。在探索提供手性 2,4-二芳基苯并二氢吡喃产物的反应范围时发现了环化途径,其核心是天然产物中发现的结构基序。
更新日期:2015-11-18
中文翻译:

手性联苯酚催化的酚、醛和硼酸酯的对映选择性多组分缩合反应
手性二醇和联苯酚催化酚、醛和烯基或芳基硼酸酯的多组分缩合反应。缩合产物以良好的产率和对映选择性形成。该反应通过醛和苯酚的初始弗里德尔-克来福特烷基化进行,产生邻醌甲基化物,然后进行对映选择性硼酸酯加成。在探索提供手性 2,4-二芳基苯并二氢吡喃产物的反应范围时发现了环化途径,其核心是天然产物中发现的结构基序。































 京公网安备 11010802027423号
京公网安备 11010802027423号