当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct electrochemical oxidation of ethanol on SOFCs: Improved carbon tolerance of Ni anode by alloying
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2015-11-18 16:10:49 Brittany Farrell, Suljo Linic
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2015-11-18 16:10:49 Brittany Farrell, Suljo Linic
|
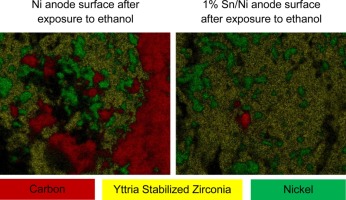
Solid oxide fuel cells (SOFCs) are electrochemical devices that convert chemical energy in fuels into electrical energy through an electrochemical oxidation process. This technology is attractive since SOFCs can in principle utilize a range of combustible fuels including hydrogen, carbon monoxide, and hydrocarbons offering higher efficiencies than conventional electricity generators with limited emission of a number of common air pollutants such as NO x and SO x . The environmental efficiency of SOFC devices can further be improved by utilizing fuels that are more carbon-neutral (e.g., biofuels such as ethanol) than conventional fossil fuels. One of the problems with employing oxygenated liquid biofuels is that conventional Ni anode electro-catalysts deactivate due to carbon deposition on the surface of the anode during the process of electocatalytic fuel oxidation. We show that the stability of Ni SOFC anode electrocatalysts during electrochemical oxidation of ethanol is significantly improved when a small amount of Sn is introduced in the electrocatalyst design. The improvement in the stability is manifested in a more stable operation and higher kinetic currents of Sn/Ni compared to Ni electrodes under identical conditions with ethanol fuel. We discuss the underlying molecular mechanisms responsible for the enhanced stability of the anodes and propose a number of guiding principles for the design of carbon-tolerant anodes for oxidation of oxygenated hydrocarbons.
中文翻译:

乙醇在SOFC上的直接电化学氧化:通过合金化提高了Ni阳极的碳耐受性
固体氧化物燃料电池(SOFC)是电化学装置,可通过电化学氧化过程将燃料中的化学能转化为电能。这项技术具有吸引力,因为SOFC原则上可以利用一系列可燃燃料,包括氢气,一氧化碳和碳氢化合物,其效率要比传统发电机高,而排放的多种常见空气污染物(例如NO x和SO x)却受到限制。。通过使用比传统化石燃料碳中性更高的燃料(例如,生物燃料,例如乙醇),可以进一步提高SOFC装置的环境效率。使用氧化的液体生物燃料的问题之一是常规的Ni阳极电催化剂由于在电催化燃料氧化过程中碳沉积在阳极表面上而失活。我们表明,当在电催化剂设计中引入少量的Sn时,Ni SOFC阳极电催化剂在乙醇电化学氧化过程中的稳定性得到了显着提高。在与乙醇燃料相同的条件下,与Ni电极相比,Sn / Ni的运行更稳定,动电流更高,从而显示出稳定性的提高。
更新日期:2015-11-19
中文翻译:

乙醇在SOFC上的直接电化学氧化:通过合金化提高了Ni阳极的碳耐受性
固体氧化物燃料电池(SOFC)是电化学装置,可通过电化学氧化过程将燃料中的化学能转化为电能。这项技术具有吸引力,因为SOFC原则上可以利用一系列可燃燃料,包括氢气,一氧化碳和碳氢化合物,其效率要比传统发电机高,而排放的多种常见空气污染物(例如NO x和SO x)却受到限制。。通过使用比传统化石燃料碳中性更高的燃料(例如,生物燃料,例如乙醇),可以进一步提高SOFC装置的环境效率。使用氧化的液体生物燃料的问题之一是常规的Ni阳极电催化剂由于在电催化燃料氧化过程中碳沉积在阳极表面上而失活。我们表明,当在电催化剂设计中引入少量的Sn时,Ni SOFC阳极电催化剂在乙醇电化学氧化过程中的稳定性得到了显着提高。在与乙醇燃料相同的条件下,与Ni电极相比,Sn / Ni的运行更稳定,动电流更高,从而显示出稳定性的提高。































 京公网安备 11010802027423号
京公网安备 11010802027423号