European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-03-23 , DOI: 10.1016/j.ejmech.2018.03.052 Linkui Zhang , Ying Zhao , Jian Wang , Donglin Yang , Chenwen Zhao , Changli Wang , Chao Ma , Maosheng Cheng
|
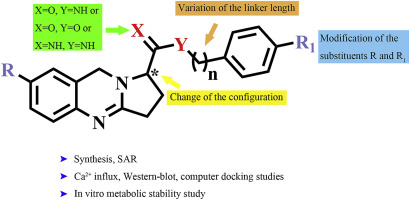
Diverse of 1,2,3,9-tetrahydropyrrolo[2,1-b]quinazoline-1-carboxylic acid derivatives were designed, synthesized and evaluated for their neuroprotective activity against NMDA-induced cytotoxicity in vitro, and 5q exhibited excellent neuroprotective activity. The compound 5q was selected for further investigation. We found that 5q could attenuate Ca2+ influx induced by NMDA, meanwhile, 5q could suppress the NR2B up-regulation and increase p-ERK1/2 expression. The molecular docking results showed that 5q might fit well in the binding pocket of 4 and interact with some key residues in the binding pocket of 1 simultaneously. Besides, 5q exhibited acceptable metabolic stability. These results suggested that 5q was a promising lead for further development of new potent and orally bioavailable NR2B-selective NMDAR antagonists.
中文翻译:

设计,合成和生物评价1,2,3,9-四氢吡咯并[2,1- b ]喹唑啉-1-羧酸衍生物作为有效的神经保护剂
设计,合成并评价了各种1,2,3,9-四氢吡咯并[2,1- b ]喹唑啉-1-羧酸衍生物对NMDA诱导的体外细胞毒性的神经保护活性,而5q表现出优异的神经保护活性。选择化合物5q进行进一步研究。我们发现5q可以减轻NMDA诱导的Ca 2+内流,同时5q可以抑制NR2B的上调并增加p -ERK1 / 2的表达。分子对接结果表明5q可能很好地适合4的结合口袋,并与5q的结合口袋中的一些关键残基相互作用。同时1个。此外,5q表现出可接受的代谢稳定性。这些结果表明5q是进一步开发新型有效的和口服可生物利用的NR2B选择性NMDAR拮抗剂的有希望的先导。































 京公网安备 11010802027423号
京公网安备 11010802027423号