Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2018-03-23 , DOI: 10.1016/j.freeradbiomed.2018.03.038
Jie Zhang 1 , Zhi-Wei Ye 1 , Shweta Singh 1 , Danyelle M Townsend 2 , Kenneth D Tew 1
|
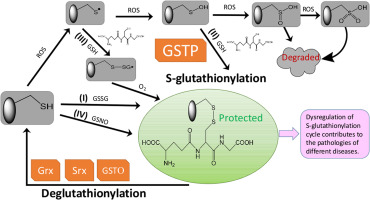
By nature of the reversibility of the addition of glutathione to low pKa cysteine residues, the post-translational modification of S-glutathionylation sanctions a cycle that can create a conduit for cell signaling events linked with cellular exposure to oxidative or nitrosative stress. The modification can also avert proteolysis by protection from over-oxidation of those clusters of target proteins that are substrates. Altered functions are associated with S-glutathionylation of proteins within the mitochondria and endoplasmic reticulum compartments, and these impact energy production and protein folding pathways. The existence of human polymorphisms of enzymes involved in the cycle (particularly glutathione S-transferase P) create a scenario for inter-individual variance in response to oxidative stress and a number of human diseases with associated aberrant S-glutathionylation have now been identified.
中文翻译:

对氧化还原调节途径中 S-谷胱甘肽循环的不断发展的理解
由于向低 pKa 半胱氨酸残基添加谷胱甘肽的可逆性,S-谷胱甘肽化的翻译后修饰会形成一个循环,该循环可以为与细胞暴露于氧化或亚硝化应激相关的细胞信号转导事件创建通道。该修饰还可以通过保护底物靶蛋白簇免受过度氧化来避免蛋白水解。功能改变与线粒体和内质网区室中蛋白质的 S-谷胱甘肽化有关,这些影响能量产生和蛋白质折叠途径。参与循环的人类酶多态性(特别是谷胱甘肽 S-转移酶 P)的存在造成了个体间对氧化应激反应存在差异的情况,并且现已发现许多与异常 S-谷胱甘肽化相关的人类疾病。

































 京公网安备 11010802027423号
京公网安备 11010802027423号