当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Phosphorylation on the Mass Spectrometry Quantification of Intact Phosphoproteins
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1021/acs.analchem.7b05246 Zhijie Wu , Timothy N Tiambeng , Wenxuan Cai , Bifan Chen , Ziqing Lin , Zachery R Gregorich , Ying Ge
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-22 00:00:00 , DOI: 10.1021/acs.analchem.7b05246 Zhijie Wu , Timothy N Tiambeng , Wenxuan Cai , Bifan Chen , Ziqing Lin , Zachery R Gregorich , Ying Ge
|
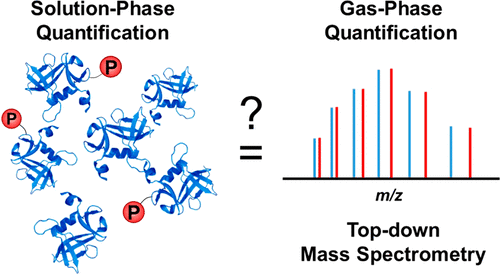
Protein phosphorylation is a ubiquitous and critical post-translational modification (PTM) involved in numerous cellular processes. Mass spectrometry (MS)-based proteomics has emerged as the preferred technology for protein identification, characterization, and quantification. Whereas ionization/detection efficiency of peptides in electrospray ionization (ESI)-MS are markedly influenced by the presence of phosphorylation, the physicochemical properties of intact proteins are assumed not to vary significantly due to the relatively smaller modification on large intact proteins. Thus, the ionization/detection efficiency of intact phosphoprotein is hypothesized not to alter appreciably for subsequent MS quantification. However, this hypothesis has never been rigorously tested. Herein, we systematically investigated the impact of phosphorylation on ESI-MS quantification of mono- and multiply phosphorylated proteins. We verified that a single phosphorylation did not appreciably affect the ESI-MS quantification of phosphoproteins as demonstrated in the enigma homolog isoform 2 (28 kDa) with monophosphorylation. Moreover, different ionization and desolvation parameters did not impact phosphoprotein quantification. In contrast to monophosphorylation, multiphosphorylation noticeably affected ESI-MS quantification of phosphoproteins likely due to differential ionization/detection efficiency between unphosphorylated and phosphorylated proteoforms as shown in the pentakis-phosphorylated β-casein (24 kDa).
中文翻译:

磷酸化对完整磷蛋白质谱定量的影响
蛋白质磷酸化是一种普遍存在且关键的翻译后修饰 (PTM),参与许多细胞过程。基于质谱 (MS) 的蛋白质组学已成为蛋白质鉴定、表征和定量的首选技术。尽管电喷雾电离 (ESI)-MS 中肽的电离/检测效率明显受到磷酸化存在的影响,但由于大的完整蛋白质的修饰相对较小,因此假定完整蛋白质的理化性质不会发生显着变化。因此,假设完整磷蛋白的电离/检测效率对于随后的 MS 定量不会发生明显改变。然而,这一假设从未经过严格的检验。在此,我们系统地研究了磷酸化对单磷酸化和多磷酸化蛋白质的 ESI-MS 定量的影响。我们验证了单磷酸化不会明显影响磷蛋白的 ESI-MS 定量,如单磷酸化的 enigma 同源异构体 2 (28 kDa) 所示。此外,不同的电离和去溶剂化参数并不影响磷蛋白的定量。与单磷酸化相反,多磷酸化显着影响磷蛋白的 ESI-MS 定量,这可能是由于未磷酸化和磷酸化蛋白质形式之间的电离/检测效率差异所致,如五磷酸化 β-酪蛋白 (24 kDa) 所示。
更新日期:2018-03-22
中文翻译:

磷酸化对完整磷蛋白质谱定量的影响
蛋白质磷酸化是一种普遍存在且关键的翻译后修饰 (PTM),参与许多细胞过程。基于质谱 (MS) 的蛋白质组学已成为蛋白质鉴定、表征和定量的首选技术。尽管电喷雾电离 (ESI)-MS 中肽的电离/检测效率明显受到磷酸化存在的影响,但由于大的完整蛋白质的修饰相对较小,因此假定完整蛋白质的理化性质不会发生显着变化。因此,假设完整磷蛋白的电离/检测效率对于随后的 MS 定量不会发生明显改变。然而,这一假设从未经过严格的检验。在此,我们系统地研究了磷酸化对单磷酸化和多磷酸化蛋白质的 ESI-MS 定量的影响。我们验证了单磷酸化不会明显影响磷蛋白的 ESI-MS 定量,如单磷酸化的 enigma 同源异构体 2 (28 kDa) 所示。此外,不同的电离和去溶剂化参数并不影响磷蛋白的定量。与单磷酸化相反,多磷酸化显着影响磷蛋白的 ESI-MS 定量,这可能是由于未磷酸化和磷酸化蛋白质形式之间的电离/检测效率差异所致,如五磷酸化 β-酪蛋白 (24 kDa) 所示。































 京公网安备 11010802027423号
京公网安备 11010802027423号