当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Flavin-Based Electron Bifurcation, A New Mechanism of Biological Energy Coupling
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.chemrev.7b00707
Wolfgang Buckel 1, 2 , Rudolf K. Thauer 1, 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1021/acs.chemrev.7b00707
Wolfgang Buckel 1, 2 , Rudolf K. Thauer 1, 2
Affiliation
|
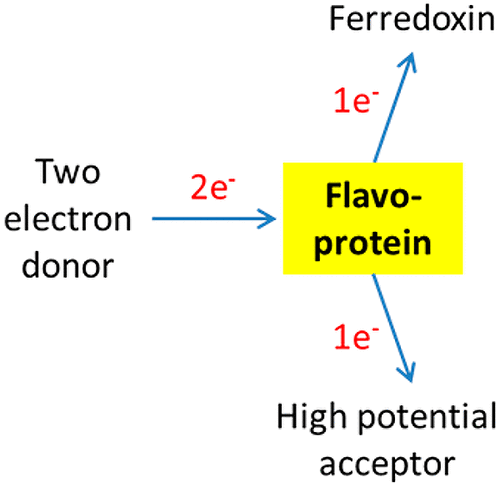
There are two types of electron bifurcation (EB), either quinone- or flavin-based (QBEB/FBEB), that involve reduction of a quinone or flavin by a two-electron transfer and two reoxidations by a high- and low-potential one-electron acceptor with a reactive semiquinone intermediate. In QBEB, the reduced low-potential acceptor (cytochrome b) is exclusively used to generate ΔμH+. In FBEB, the “energy-rich” low-potential reduced ferredoxin or flavodoxin has dual function. It can give rise to ΔμH+/Na+ via a ferredoxin:NAD reductase (Rnf) or ferredoxin:proton reductase (Ech) or conducts difficult reductions such as CO2 to CO. The QBEB membrane complexes are similar in structure and function and occur in all domains of life. In contrast, FBEB complexes are soluble and occur only in strictly anaerobic bacteria and archaea (FixABCX being an exception). The FBEB complexes constitute a group consisting of four unrelated families that contain (1) electron-transferring flavoproteins (EtfAB), (2) NAD(P)H dehydrogenase (NuoF homologues), (3) heterodisulfide reductase (HdrABC) or HdrABC homologues, and (4) NADH-dependent ferredoxin:NADP reductase (NfnAB). The crystal structures and electron transport of EtfAB-butyryl-CoA dehydrogenase and NfnAB are compared with those of complex III of the respiratory chain (cytochrome bc1), whereby unexpected common features have become apparent.
中文翻译:

基于黄素的电子分叉,一种生物能耦合的新机理
电子分叉(EB)有两种类型,基于醌或黄素的(QBEB / FBEB),涉及通过两电子转移还原醌或黄素,以及通过高电势和低电势进行两次再氧化-电子受体与反应性半醌中间体。在QBEB中,还原的低电位受体(细胞色素b)专门用于产生ΔμH +。在FBEB中,“能量丰富”的低电势还原铁氧还蛋白或黄酮毒素具有双重功能。它可以通过铁氧还蛋白:NAD还原酶(Rnf)或铁氧还蛋白:质子还原酶(Ech)产生ΔμH + / Na +或难于还原,例如CO 2QBEB膜复合物的结构和功能相似,并存在于生活的所有领域。相反,FBEB复合物是可溶的,仅在严格厌氧的细菌和古细菌中存在(FixABCX是例外)。FBEB复合物由四个不相关的家族组成,它们包含(1)电子转移黄素(EtfAB),(2)NAD(P)H脱氢酶(NuoF同源物),(3)异二硫键还原酶(HdrABC)或HdrABC同源物, (4)NADH依赖性铁氧还蛋白:NADP还原酶(NfnAB)。将EtfAB-丁酰-CoA脱氢酶和NfnAB的晶体结构和电子传输与呼吸链的复合物III(细胞色素bc 1)的晶体结构和电子传输进行了比较,从而出乎意料的共同特征变得很明显。
更新日期:2018-03-21
中文翻译:

基于黄素的电子分叉,一种生物能耦合的新机理
电子分叉(EB)有两种类型,基于醌或黄素的(QBEB / FBEB),涉及通过两电子转移还原醌或黄素,以及通过高电势和低电势进行两次再氧化-电子受体与反应性半醌中间体。在QBEB中,还原的低电位受体(细胞色素b)专门用于产生ΔμH +。在FBEB中,“能量丰富”的低电势还原铁氧还蛋白或黄酮毒素具有双重功能。它可以通过铁氧还蛋白:NAD还原酶(Rnf)或铁氧还蛋白:质子还原酶(Ech)产生ΔμH + / Na +或难于还原,例如CO 2QBEB膜复合物的结构和功能相似,并存在于生活的所有领域。相反,FBEB复合物是可溶的,仅在严格厌氧的细菌和古细菌中存在(FixABCX是例外)。FBEB复合物由四个不相关的家族组成,它们包含(1)电子转移黄素(EtfAB),(2)NAD(P)H脱氢酶(NuoF同源物),(3)异二硫键还原酶(HdrABC)或HdrABC同源物, (4)NADH依赖性铁氧还蛋白:NADP还原酶(NfnAB)。将EtfAB-丁酰-CoA脱氢酶和NfnAB的晶体结构和电子传输与呼吸链的复合物III(细胞色素bc 1)的晶体结构和电子传输进行了比较,从而出乎意料的共同特征变得很明显。

































 京公网安备 11010802027423号
京公网安备 11010802027423号