当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures of the Catalytic Domain of Bacterial Primase DnaG in Complexes with DNA Provide Insight into Key Priming Events
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1021/acs.biochem.8b00036
Caixia Hou 1 , Tapan Biswas 2 , Oleg V. Tsodikov 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-20 00:00:00 , DOI: 10.1021/acs.biochem.8b00036
Caixia Hou 1 , Tapan Biswas 2 , Oleg V. Tsodikov 1
Affiliation
|
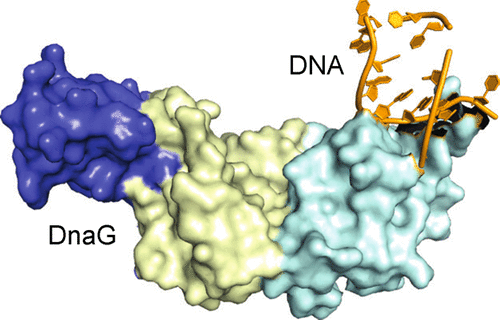
Bacterial primase DnaG is an essential nucleic acid polymerase that generates primers for replication of chromosomal DNA. The mechanism of DnaG remains unclear due to the paucity of structural information on DnaG in complexes with other replisome components. Here we report the first crystal structures of noncovalent DnaG–DNA complexes, obtained with the RNA polymerase domain of Mycobacterium tuberculosis DnaG and various DNA ligands. One structure, obtained with ds DNA, reveals interactions with DnaG as it slides on ds DNA and suggests how DnaG binds template for primer synthesis. In another structure, DNA in the active site of DnaG mimics the primer, providing insight into mechanisms for the nucleotide transfer and DNA translocation. In conjunction with the recent cryo-EM structure of the bacteriophage T7 replisome, this study yields a model for primer elongation and hand-off to DNA polymerase.
中文翻译:

具有DNA的复合物中细菌引物DnaG催化结构域的结构提供了对关键引物事件的洞察力
细菌引物酶DnaG是必不可少的核酸聚合酶,可产生用于复制染色体DNA的引物。由于与其他复制体成分复合的DnaG结构信息很少,DnaG的机制仍不清楚。在这里,我们报告了通过结核分枝杆菌的RNA聚合酶结构域获得的非共价DnaG–DNA复合物的第一个晶体结构。DnaG和各种DNA配体。用ds DNA获得的一种结构揭示了DnaG在ds DNA上滑动时与DnaG的相互作用,并暗示了DnaG如何结合模板进行引物合成。在另一种结构中,DnaG活性位点中的DNA模仿了引物,从而深入了解了核苷酸转移和DNA易位的机制。结合噬菌体T7复制体的最新cryo-EM结构,该研究产生了引物延伸和移交给DNA聚合酶的模型。
更新日期:2018-03-20
中文翻译:

具有DNA的复合物中细菌引物DnaG催化结构域的结构提供了对关键引物事件的洞察力
细菌引物酶DnaG是必不可少的核酸聚合酶,可产生用于复制染色体DNA的引物。由于与其他复制体成分复合的DnaG结构信息很少,DnaG的机制仍不清楚。在这里,我们报告了通过结核分枝杆菌的RNA聚合酶结构域获得的非共价DnaG–DNA复合物的第一个晶体结构。DnaG和各种DNA配体。用ds DNA获得的一种结构揭示了DnaG在ds DNA上滑动时与DnaG的相互作用,并暗示了DnaG如何结合模板进行引物合成。在另一种结构中,DnaG活性位点中的DNA模仿了引物,从而深入了解了核苷酸转移和DNA易位的机制。结合噬菌体T7复制体的最新cryo-EM结构,该研究产生了引物延伸和移交给DNA聚合酶的模型。

































 京公网安备 11010802027423号
京公网安备 11010802027423号