当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An efficient approach to 4-chloro quinolines via TMSCl-mediated cascade cyclization of ortho-propynol phenyl azides†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8qo00162f Xian-Rong Song 1, 2, 3, 4, 5 , Ren Li 1, 2, 3, 4, 5 , Haixin Ding 1, 2, 3, 4, 5 , Xi Chen 1, 2, 3, 4, 5 , Tao Yang 1, 2, 3, 4, 5 , Jiang Bai 1, 2, 3, 4, 5 , Qiang Xiao 1, 2, 3, 4, 5 , Yong-Min Liang 5, 6, 7, 8
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-21 00:00:00 , DOI: 10.1039/c8qo00162f Xian-Rong Song 1, 2, 3, 4, 5 , Ren Li 1, 2, 3, 4, 5 , Haixin Ding 1, 2, 3, 4, 5 , Xi Chen 1, 2, 3, 4, 5 , Tao Yang 1, 2, 3, 4, 5 , Jiang Bai 1, 2, 3, 4, 5 , Qiang Xiao 1, 2, 3, 4, 5 , Yong-Min Liang 5, 6, 7, 8
Affiliation
|
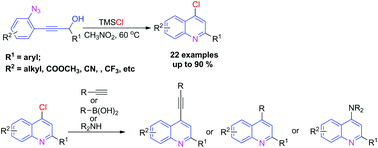
A novel and efficient strategy for the synthesis of 4-chloro quinolines via the TMSCl-mediated cascade cyclization of easily prepared ortho-propynol phenyl azides is developed. This reaction proceeds smoothly in moderate to excellent yields with a wide range of substrate compatibility. TMSCl acted not only as a promoter, but also as a chloro source in this transformation. Moreover, the obtained 4-chloro quinolines could be further derivated in an array of palladium-catalyzed coupling and nucleophilic substitution reactions to construct potential biologically active and pharmaceutical molecules.
中文翻译:

通过TMSCl介导的邻丙炔酚苯叠氮化物级联环化反应制备 4-氯喹啉的有效方法†
通过易于制备的邻-丙炔醇苯基叠氮化物的TMCSI介导的级联环化反应,开发了一种新颖而有效的合成4-氯喹啉的策略。该反应以中等至极好的收率顺利进行,并具有广泛的底物相容性。TMSC1在该转化中不仅充当启动子,而且充当氯源。而且,获得的4-氯喹啉可以在钯催化的偶联和亲核取代反应的阵列中进一步衍生,以构建潜在的生物活性和药物分子。
更新日期:2018-03-21
中文翻译:

通过TMSCl介导的邻丙炔酚苯叠氮化物级联环化反应制备 4-氯喹啉的有效方法†
通过易于制备的邻-丙炔醇苯基叠氮化物的TMCSI介导的级联环化反应,开发了一种新颖而有效的合成4-氯喹啉的策略。该反应以中等至极好的收率顺利进行,并具有广泛的底物相容性。TMSC1在该转化中不仅充当启动子,而且充当氯源。而且,获得的4-氯喹啉可以在钯催化的偶联和亲核取代反应的阵列中进一步衍生,以构建潜在的生物活性和药物分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号