当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Fluoren-9-ones and Ladder-Type Oligo-p-phenylene Cores via Pd-Catalyzed Carbonylative Multiple C–C Bond Formation
Organic Letters ( IF 4.9 ) Pub Date : 2015-04-20 00:00:00 , DOI: 10.1021/acs.orglett.5b00680
Juan Song 1 , Fuliang Wei 1 , Wei Sun 1 , Ke Li 2 , Yanan Tian 2 , Chao Liu 2 , Yali Li 1 , Linghai Xie 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-04-20 00:00:00 , DOI: 10.1021/acs.orglett.5b00680
Juan Song 1 , Fuliang Wei 1 , Wei Sun 1 , Ke Li 2 , Yanan Tian 2 , Chao Liu 2 , Yali Li 1 , Linghai Xie 1
Affiliation
|
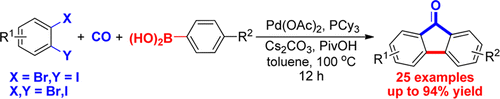
A new route to various substituted fluoren-9-ones has been developed via an efficient Pd-catalyzed carbonylative multiple C–C bond formation. Under a CO atmosphere, using commercially available aryl halides and arylboronic acids as substrates, this three-component reaction proceeded smoothly in moderate to excellent yields with good functional-group compatibility. The mechanistic investigations suggested a sequential process for the reaction that forms o-bromobiaryls in the first stage followed by a cyclocarbonylation reaction. This chemistry has been successfully extended to construct ladder-type oligo-p-phenylene cores.
中文翻译:

通过Pd催化的羰基化多个C–C键的形成,合成芴9-酮和梯型低聚对苯撑核
通过有效的Pd催化的羰基化多个C–C键的形成,已开发出一种新的途径来取代各种9-9取代的芴。在CO气氛下,使用可商购的芳基卤化物和芳基硼酸作为底物,该三组分反应以中等至优异的收率顺利进行,并具有良好的官能团相容性。机理研究表明,反应的顺序过程是在第一步中形成邻溴代双芳基,然后进行环羰基化反应。该化学方法已成功地扩展到构建梯型低聚对亚苯基核心。
更新日期:2015-04-20
中文翻译:

通过Pd催化的羰基化多个C–C键的形成,合成芴9-酮和梯型低聚对苯撑核
通过有效的Pd催化的羰基化多个C–C键的形成,已开发出一种新的途径来取代各种9-9取代的芴。在CO气氛下,使用可商购的芳基卤化物和芳基硼酸作为底物,该三组分反应以中等至优异的收率顺利进行,并具有良好的官能团相容性。机理研究表明,反应的顺序过程是在第一步中形成邻溴代双芳基,然后进行环羰基化反应。该化学方法已成功地扩展到构建梯型低聚对亚苯基核心。

































 京公网安备 11010802027423号
京公网安备 11010802027423号