当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of 2-Arylbenzo[4,5]thieno[2,3-d]thiazole Skeleton via CuCl/S-Mediated Three-Component Reaction.
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.orglett.9b04206 Wei Zhang 1 , Shanqing Tao 1 , Huaibin Ge 1 , Qiao Li 1 , Zhenkang Ai 1 , Xiaoxian Li 1 , Beibei Zhang 1 , Fengxia Sun 2 , Xiaqing Xu 3 , Yunfei Du 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.orglett.9b04206 Wei Zhang 1 , Shanqing Tao 1 , Huaibin Ge 1 , Qiao Li 1 , Zhenkang Ai 1 , Xiaoxian Li 1 , Beibei Zhang 1 , Fengxia Sun 2 , Xiaqing Xu 3 , Yunfei Du 1
Affiliation
|
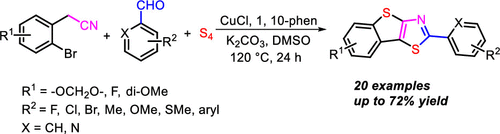
An exclusive thiophene-fused polycyclic π-conjugated 2-arylbenzo[4,5]thieno[2,3-d]thiazole skeleton was constructed via a one-pot CuCl-mediated three-component reaction, using 2-(2-bromophenyl)acetonitrile and aromatic aldehydes as substrates and elemental sulfur as sulfur source in the presence of K2CO3 and 1,10-phen in DMSO. A plausible reaction mechanism was proposed, which involved formation of benzo[b]thiophen-2-amines through cyclization of 2-bromophenyl acetonitrile and sulfur, and subsequent intramolecular condensation/dehydrogenation with aromatic aldehydes.
中文翻译:

通过CuCl / S介导的三组分反应构建2-芳基苯并[4,5]噻吩并[2,3-d]噻唑骨架。
使用2-(2-溴苯基)通过一锅CuCl介导的三组分反应构建排他性噻吩稠合的多环π共轭2-芳基苯并[4,5]噻吩并[2,3-d]噻唑骨架在DMSO中存在K2CO3和1,10-phen的条件下,以乙腈和芳族醛为底物,以元素硫为硫源。提出了一种可能的反应机理,该机理涉及通过2-溴苯基乙腈和硫的环化形成苯并[b]噻吩-2-胺,然后进行分子内的缩合/与芳香族醛的脱氢反应。
更新日期:2020-01-02
中文翻译:

通过CuCl / S介导的三组分反应构建2-芳基苯并[4,5]噻吩并[2,3-d]噻唑骨架。
使用2-(2-溴苯基)通过一锅CuCl介导的三组分反应构建排他性噻吩稠合的多环π共轭2-芳基苯并[4,5]噻吩并[2,3-d]噻唑骨架在DMSO中存在K2CO3和1,10-phen的条件下,以乙腈和芳族醛为底物,以元素硫为硫源。提出了一种可能的反应机理,该机理涉及通过2-溴苯基乙腈和硫的环化形成苯并[b]噻吩-2-胺,然后进行分子内的缩合/与芳香族醛的脱氢反应。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号