当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing Symmetrical Tetraphenyl‐pyrrole‐2‐yl Squaraines Through an Improved Synthesis of 2,4‐Diphenylpyrrole
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-02 , DOI: 10.1002/slct.201904581 Arkadiusz Gut 1 , Maria Stosz 1 , Łukasz Łapok 1 , Katarzyna M. Stadnicka 1 , Maria Nowakowska 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-01-02 , DOI: 10.1002/slct.201904581 Arkadiusz Gut 1 , Maria Stosz 1 , Łukasz Łapok 1 , Katarzyna M. Stadnicka 1 , Maria Nowakowska 1
Affiliation
|
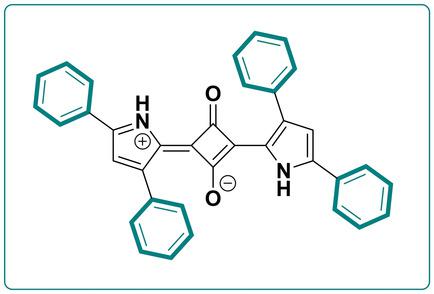
Synthesis of tetraphenyl‐pyrrole‐2‐yl squaraines is introduced for the first time. An improved, reproducible and straightforward method for the preparation of 2,4‐diphenylpyrrole‐a key structural synthon for the preparation of the squaraines in question‐is reported. Both, squaraine dyes and all intermediates were fully characterized using spectroscopic methods, viz. 1H NMR, 13C NMR, 2D NMR (COSY, HSQC, HMBC), FT‐ATR‐IR, ESI‐HRMS. The structures of tetraphenyl‐pyrrol‐2‐yl squaraines were unambiguously confirmed by single X‐ray analysis. The squaraines studied exhibit an intense absorption bands spanning from ca. 600 to 700 nm, entering to the spectral region, which is highly sought after for biomedical applications and advanced material technologies.
中文翻译:

通过改进的2,4-二苯并吡咯合成方法获得对称的四苯并吡咯-2-基鱿鱼
首次介绍了四苯基吡咯-2-基方酸的合成。据报道,一种改进的,可重复的和直接的制备2,4-二苯基吡咯的方法是一种关键的结构合成子,用于制备相关的qua类。方酸染料和所有中间体均使用分光镜方法进行了充分表征。1 H NMR,13 C NMR,2D NMR(COSY,HSQC,HMBC),FT-ATR-IR,ESI-HRMS。单次X射线分析清楚地证实了四苯基吡咯-2-基扁豆的结构。所研究的方类动物表现出从ca跨越的强烈吸收带。600到700 nm,进入光谱区域,这在生物医学应用和先进的材料技术中非常受追捧。
更新日期:2020-01-02
中文翻译:

通过改进的2,4-二苯并吡咯合成方法获得对称的四苯并吡咯-2-基鱿鱼
首次介绍了四苯基吡咯-2-基方酸的合成。据报道,一种改进的,可重复的和直接的制备2,4-二苯基吡咯的方法是一种关键的结构合成子,用于制备相关的qua类。方酸染料和所有中间体均使用分光镜方法进行了充分表征。1 H NMR,13 C NMR,2D NMR(COSY,HSQC,HMBC),FT-ATR-IR,ESI-HRMS。单次X射线分析清楚地证实了四苯基吡咯-2-基扁豆的结构。所研究的方类动物表现出从ca跨越的强烈吸收带。600到700 nm,进入光谱区域,这在生物医学应用和先进的材料技术中非常受追捧。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号