当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silver-Catalyzed Annulation of Arynes with Nitriles for Synthesis of Structurally Diverse Quinazolines.
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.orglett.9b04395 Sourav Ghorai 1 , Yongjia Lin 2 , Yuanzhi Xia 2 , Donald J Wink 1 , Daesung Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-12-30 , DOI: 10.1021/acs.orglett.9b04395 Sourav Ghorai 1 , Yongjia Lin 2 , Yuanzhi Xia 2 , Donald J Wink 1 , Daesung Lee 1
Affiliation
|
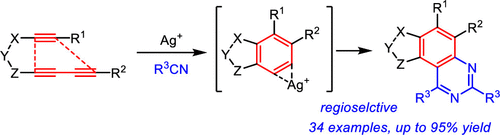
An efficient silver catalyzed annulation reaction of aryne with nitriles to generate quinazolines is described. Arynes generated from triynes or tetraynes through a hexadehydro Diels-Alder reaction readily participated in an [A + 2B] mode of annulation with nitriles in the presence of AgSbF6 catalyst. The mechanism was explored by DFT calculations, which supports the silver-catalyzed formation of nitrilium ion as a key intermediate. This annulation generates an array of polysubstituted novel quinazoline derivatives with excellent regioselectivity.
中文翻译:

银催化的带有丁腈的芳烃环用于合成结构多样的喹唑啉。
描述了炔烃与腈的有效银催化环化反应以生成喹唑啉。在AgSbF6催化剂的存在下,通过六氢Diels-Alder反应由三炔或四炔生成的芳烃很容易参与腈的环化[A + 2B]模式。通过DFT计算探索了该机理,该机理支持银催化的腈离子作为关键中间体的形成。这种成环反应产生了具有优良区域选择性的多取代的新型喹唑啉衍生物阵列。
更新日期:2019-12-31
中文翻译:

银催化的带有丁腈的芳烃环用于合成结构多样的喹唑啉。
描述了炔烃与腈的有效银催化环化反应以生成喹唑啉。在AgSbF6催化剂的存在下,通过六氢Diels-Alder反应由三炔或四炔生成的芳烃很容易参与腈的环化[A + 2B]模式。通过DFT计算探索了该机理,该机理支持银催化的腈离子作为关键中间体的形成。这种成环反应产生了具有优良区域选择性的多取代的新型喹唑啉衍生物阵列。































 京公网安备 11010802027423号
京公网安备 11010802027423号