当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides bearing heteroaromatic rings as novel antibacterial agents: Design, synthesis, biological evaluation and target identification.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.ejmech.2019.112022 Wenjie Xue 1 , Xueyao Li 1 , Guixing Ma 2 , Hongmin Zhang 2 , Ya Chen 3 , Johannes Kirchmair 4 , Jie Xia 1 , Song Wu 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-12-30 , DOI: 10.1016/j.ejmech.2019.112022 Wenjie Xue 1 , Xueyao Li 1 , Guixing Ma 2 , Hongmin Zhang 2 , Ya Chen 3 , Johannes Kirchmair 4 , Jie Xia 1 , Song Wu 1
Affiliation
|
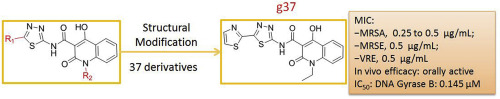
Due to the occurrence of antibiotic resistance, bacterial infectious diseases have become a serious threat to public health. To overcome antibiotic resistance, novel antibiotics are urgently needed. N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides are a potential new class of antibacterial agents, as one of its derivatives was identified as an antibacterial agent against S. aureus. However, no potency-directed structural optimization has been performed. In this study, we designed and synthesized 37 derivatives, and evaluated their antibacterial activity against S. aureus ATCC29213, which led to the identification of ten potent antibacterial agents with minimum inhibitory concentration (MIC) values below 1 μg/mL. Next, we performed bacterial growth inhibition assays against a panel of drug-resistant clinical isolates, including methicillin-resistant S. aureus, and cytotoxicity assays with HepG2 and HUVEC cells. One of the tested compounds named 1-ethyl-4-hydroxy-2-oxo-N-(5-(thiazol-2-yl)-1,3,4-thiadiazol-2-yl)-1,2-dihydroquinoline-3-carboxamide (g37) showed 2 to 128-times improvement compared with vancomycin in term of antibacterial potency against the tested strains (MICs: 0.25-1 μg/mL vs. 1-64 μg/mL) and an optimal selective toxicity (HepG2/MRSA, 110.6 to 221.2; HUVEC/MRSA, 77.6-155.2). Further, comprehensive evaluation indicated that g37 did not induce resistance development of MRSA over 20 passages, and it has been confirmed as a bactericidal, metabolically stable, orally active antibacterial agent. More importantly, we have identified the S. aureus DNA gyrase B as its potential target and proposed a potential binding mode by molecular docking. Taken together, the present work reports the most potent derivative of this chemical series (g37) and uncovers its potential target, which lays a solid foundation for further lead optimization facilitated by the structure-based drug design technique.
中文翻译:

带有杂芳环的N-噻二唑-4-羟基-2-喹诺酮-3-羧酰胺作为新型抗菌剂:设计,合成,生物学评估和目标识别。
由于发生抗生素抗性,细菌感染性疾病已成为对公共健康的严重威胁。为了克服抗生素抗性,迫切需要新型抗生素。N-噻二唑-4-羟基-2-喹诺酮-3-羧酰胺是一类潜在的新型抗菌剂,因为其衍生物之一被确定为针对金黄色葡萄球菌的抗菌剂。但是,尚未执行效能导向的结构优化。在这项研究中,我们设计和合成了37种衍生物,并评估了它们对金黄色葡萄球菌ATCC29213的抗菌活性,从而鉴定出十种有效抑菌剂,其最低抑菌浓度(MIC)值均低于1μg/ mL。接下来,我们针对一组抗药性临床分离株进行了细菌生长抑制测定,包括耐甲氧西林的金黄色葡萄球菌,以及对HepG2和HUVEC细胞的细胞毒性测定。一种被测试的化合物,称为1-乙基-4-羟基-2-氧代-N-(5-(噻唑-2-基)-1,3,4-噻二唑-2-基)-1,2-二氢喹啉-与万古霉素相比,3-羧酰胺(g37)对被测菌株的抗菌力(MIC:0.25-1μg/ mL对1-64μg/ mL)表现出2至128倍的改善,并且具有最佳的选择性毒性(HepG2 /MRSA,110.6至221.2; HUVEC / MRSA,77.6-155.2)。此外,综合评估表明,g37不会在20代内诱导MRSA产生耐药性,并且已确认它是杀菌,代谢稳定,口服活性的抗菌剂。更重要的是,我们已经确定了金黄色葡萄球菌DNA促旋酶B作为其潜在的靶标,并通过分子对接提出了一种潜在的结合方式。
更新日期:2019-12-31
中文翻译:

带有杂芳环的N-噻二唑-4-羟基-2-喹诺酮-3-羧酰胺作为新型抗菌剂:设计,合成,生物学评估和目标识别。
由于发生抗生素抗性,细菌感染性疾病已成为对公共健康的严重威胁。为了克服抗生素抗性,迫切需要新型抗生素。N-噻二唑-4-羟基-2-喹诺酮-3-羧酰胺是一类潜在的新型抗菌剂,因为其衍生物之一被确定为针对金黄色葡萄球菌的抗菌剂。但是,尚未执行效能导向的结构优化。在这项研究中,我们设计和合成了37种衍生物,并评估了它们对金黄色葡萄球菌ATCC29213的抗菌活性,从而鉴定出十种有效抑菌剂,其最低抑菌浓度(MIC)值均低于1μg/ mL。接下来,我们针对一组抗药性临床分离株进行了细菌生长抑制测定,包括耐甲氧西林的金黄色葡萄球菌,以及对HepG2和HUVEC细胞的细胞毒性测定。一种被测试的化合物,称为1-乙基-4-羟基-2-氧代-N-(5-(噻唑-2-基)-1,3,4-噻二唑-2-基)-1,2-二氢喹啉-与万古霉素相比,3-羧酰胺(g37)对被测菌株的抗菌力(MIC:0.25-1μg/ mL对1-64μg/ mL)表现出2至128倍的改善,并且具有最佳的选择性毒性(HepG2 /MRSA,110.6至221.2; HUVEC / MRSA,77.6-155.2)。此外,综合评估表明,g37不会在20代内诱导MRSA产生耐药性,并且已确认它是杀菌,代谢稳定,口服活性的抗菌剂。更重要的是,我们已经确定了金黄色葡萄球菌DNA促旋酶B作为其潜在的靶标,并通过分子对接提出了一种潜在的结合方式。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号