Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Mechanism of the Sn2Fe Anode in Lithium-Ion Batteries.
ACS Omega ( IF 3.7 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsomega.9b02417 Zhixin Dong 1 , Qi Wang 1 , Ruibo Zhang 1 , Natasha A Chernova 1 , Fredrick Omenya 1 , Dongsheng Ji 1 , M Stanley Whittingham 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-12-18 , DOI: 10.1021/acsomega.9b02417 Zhixin Dong 1 , Qi Wang 1 , Ruibo Zhang 1 , Natasha A Chernova 1 , Fredrick Omenya 1 , Dongsheng Ji 1 , M Stanley Whittingham 1
Affiliation
|
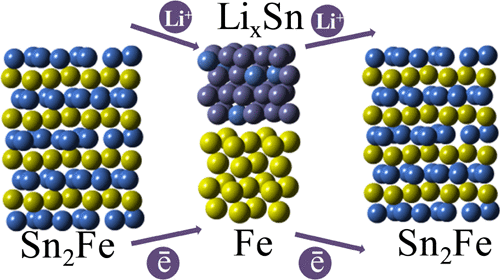
Sn2Fe anode materials were synthesized by a solvothermal route, and their electrochemical performance and reaction mechanism were evaluated. The structural evolution in the first two lithium cycles was investigated by X-ray absorption spectroscopy (XAS), synchrotron X-ray diffraction (XRD), and magnetic studies. In the first cycle, progressive alloying of Sn with Li accompanied by metallic iron displacement occurs upon lithiation, and the delithiation proceeds by Li x Sn dealloying and recovery of the Sn2Fe phase. In the second cycle, both XRD and XAS identify Li-Sn alloying at earlier lithiation stages than in the first cycle, with low-Li-content alloys evident in the beginning of the lithiation process. In the fully lithiated state, XAS analysis reveals higher coordination numbers in both the Li x Sn and Fe phases, which points toward more complete reaction and higher crystallinity of the products. Upon second delithiation, the Sn2Fe phase is generally reformed as evidenced by XRD. However, XAS indicates somewhat reduced Sn-Fe coordination and shorter Fe-Fe distance, which indicates incomplete reconversion and metallic Fe retention, which is also evident in the magnetic studies. Thus, a combination of long-range (XRD, magnetic) and local (XAS) techniques has revealed differences between the first and the second Li cycles relevant to the understanding of the capacity fading mechanisms.
中文翻译:

锂离子电池中Sn2Fe阳极的反应机理。
通过溶剂热法合成了Sn2Fe阳极材料,并对其电化学性能和反应机理进行了评价。通过X射线吸收光谱(XAS),同步加速器X射线衍射(XRD)和磁学研究了前两个锂循环中的结构演变。在第一个循环中,在锂化过程中会发生Sn与Li的渐进合金化,伴随着金属铁的置换,并且通过Li x Sn脱合金和Sn2Fe相的恢复进行脱锂。在第二个循环中,XRD和XAS都在比第一个循环更早的锂化阶段识别出Li-Sn合金化,并且在锂化过程的开始就明显地发现了低Li含量的合金。在完全锂化状态下,XAS分析显示出Li x Sn和Fe相中的配位数更高,这表明产物更完全的反应和更高的结晶度。在第二次脱锂时,如XRD所示,通常对Sn2Fe相进行重整。然而,XAS指示Sn-Fe配位有所降低,Fe-Fe距离更短,这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。
更新日期:2019-12-31
中文翻译:

锂离子电池中Sn2Fe阳极的反应机理。
通过溶剂热法合成了Sn2Fe阳极材料,并对其电化学性能和反应机理进行了评价。通过X射线吸收光谱(XAS),同步加速器X射线衍射(XRD)和磁学研究了前两个锂循环中的结构演变。在第一个循环中,在锂化过程中会发生Sn与Li的渐进合金化,伴随着金属铁的置换,并且通过Li x Sn脱合金和Sn2Fe相的恢复进行脱锂。在第二个循环中,XRD和XAS都在比第一个循环更早的锂化阶段识别出Li-Sn合金化,并且在锂化过程的开始就明显地发现了低Li含量的合金。在完全锂化状态下,XAS分析显示出Li x Sn和Fe相中的配位数更高,这表明产物更完全的反应和更高的结晶度。在第二次脱锂时,如XRD所示,通常对Sn2Fe相进行重整。然而,XAS指示Sn-Fe配位有所降低,Fe-Fe距离更短,这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。这表明不完全转化和金属Fe保留,这在磁性研究中也很明显。因此,远程(XRD,电磁)技术和局部(XAS)技术的结合揭示了第一和第二次Li循环之间的差异,这与对容量衰减机制的理解有关。






























 京公网安备 11010802027423号
京公网安备 11010802027423号