当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature of Interactions between Epoxides in Graphene Oxide
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jpcc.9b10262 Pattath D. Pancharatna 1 , Gaurav Jhaa 1 , Musiri M. Balakrishnarajan 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-08 , DOI: 10.1021/acs.jpcc.9b10262 Pattath D. Pancharatna 1 , Gaurav Jhaa 1 , Musiri M. Balakrishnarajan 1
Affiliation
|
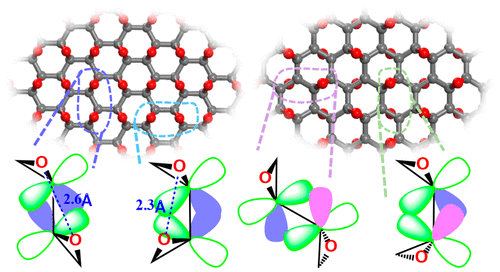
Graphene oxide exhibits extensive disorder with a multitude of functional groups and holes making its structure an abstract concept. Multiple structural models make it a dark horse and hamper its utility despite its synthetic ease, maneuverability, and promising applications. Here we probe the impact of the epoxidation process, which is arguably the first kinetic step in graphene oxidation, to identify the induced vulnerabilities of its backbone and to cognize possible pathways for further oxidation. Probing the topological and geometrical variations in the distribution of epoxide on the graphene lattice, we find that the conformational entropy, driven by the combinatorial growth of isomers, aids and abets disorder. Graph theoretical enumeration gives 16 distinct epoxide environments within symmetrically equivalent epoxides. Their stability is primarily influenced by the steric repulsion between the oxygen lone pairs and overlap compatibility of the interepoxy C–C bond. Avoiding steric repulsion either by topology or by equatorial splaying of oxygens leads to stability, without which the network is weakened either by elongation of C–C bonds or by axial splaying of oxygens leading to uneven C–O bonds. The proposed unzipping of the underlying epoxy C–C bond through the cooperativity of strain from three-membered rings and conjugative stabilization from residual sp2 character are found to be incongruous. With an improved bonding model of epoxide, we account for the observed variations in C–C bond lengths and energetics of epoxides in different environments that facilitate strategic mechanistic control for further oxidation.
中文翻译:

氧化石墨烯中环氧之间相互作用的性质
氧化石墨烯表现出广泛的紊乱,具有大量的官能团和孔洞,使其结构成为一个抽象概念。多种结构模型使它成为一匹黑马,并且尽管其综合性的易用性,可操作性和广阔的应用前景也妨碍了它的实用性。在这里,我们探讨了环氧化过程(可以说是石墨烯氧化的第一个动力学步骤)的影响,以确定其骨架的诱导脆弱性,并认识到进一步氧化的可能途径。探究石墨烯晶格上环氧分布的拓扑和几何变化,我们发现构象熵是由异构体,助剂和教disorder症的组合生长驱动的。图的理论枚举给出了在对称等效环氧化物内的16个不同的环氧化物环境。它们的稳定性主要受氧孤对之间的空间排斥力和环氧基间CC键的重叠相容性的影响。避免由于拓扑结构或氧的赤道张开而产生的空间排斥会导致稳定性,如果不这样做,则由于C–C键的延长或氧的轴向张开会导致C–O键不均匀,从而削弱了网络。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键 否则,网络会由于C–C键的延长或氧的轴向张开而导致不均匀的C–O键而被削弱。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键 否则,网络可能会因C–C键的延长或氧的轴向张开而变弱,从而导致C–O键不均匀。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键发现2个字符不一致。通过改进的环氧化物键合模型,我们可以解释在不同环境中观察到的CC键长变化和环氧化物的能级,这有利于进一步氧化的策略性机械控制。
更新日期:2020-01-08
中文翻译:

氧化石墨烯中环氧之间相互作用的性质
氧化石墨烯表现出广泛的紊乱,具有大量的官能团和孔洞,使其结构成为一个抽象概念。多种结构模型使它成为一匹黑马,并且尽管其综合性的易用性,可操作性和广阔的应用前景也妨碍了它的实用性。在这里,我们探讨了环氧化过程(可以说是石墨烯氧化的第一个动力学步骤)的影响,以确定其骨架的诱导脆弱性,并认识到进一步氧化的可能途径。探究石墨烯晶格上环氧分布的拓扑和几何变化,我们发现构象熵是由异构体,助剂和教disorder症的组合生长驱动的。图的理论枚举给出了在对称等效环氧化物内的16个不同的环氧化物环境。它们的稳定性主要受氧孤对之间的空间排斥力和环氧基间CC键的重叠相容性的影响。避免由于拓扑结构或氧的赤道张开而产生的空间排斥会导致稳定性,如果不这样做,则由于C–C键的延长或氧的轴向张开会导致C–O键不均匀,从而削弱了网络。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键 否则,网络会由于C–C键的延长或氧的轴向张开而导致不均匀的C–O键而被削弱。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键 否则,网络可能会因C–C键的延长或氧的轴向张开而变弱,从而导致C–O键不均匀。通过三元环应变的协同作用和残余sp的共轭稳定作用,建议解开潜在的环氧CC键发现2个字符不一致。通过改进的环氧化物键合模型,我们可以解释在不同环境中观察到的CC键长变化和环氧化物的能级,这有利于进一步氧化的策略性机械控制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号