当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Principles and Microkinetic Study on the Mechanism for Ammonia Synthesis Using Ru-Loaded Hydride Catalyst
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jpcc.9b10850
Takuya Nakao 1 , Tomofumi Tada 1 , Hideo Hosono 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-01-10 , DOI: 10.1021/acs.jpcc.9b10850
Takuya Nakao 1 , Tomofumi Tada 1 , Hideo Hosono 1
Affiliation
|
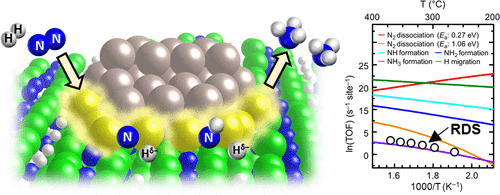
Ru-loaded hydride is an efficient catalyst for ammonia (NH3) synthesis under mild conditions. Metal hydrides such as Ca2NH with surface anionic electrons at hydrogen vacancies (Ca2NH1–xex–) function well as active catalytic support materials for Ru. The resultant catalysts exhibit good performance for NH3 synthesis with a large reduction of the apparent activation energy and the suppression of hydrogen poisoning of Ru. However, the reaction mechanism and the rate-determining step (RDS) have not yet been clarified from a microscopic viewpoint. Here, we have successfully reproduced the experimental results of NH3 synthesis by microkinetic modeling using density functional theory (DFT) calculations. Three essential mechanisms were identified: (i) the promotion of nitrogen cleavage with electron injection from Ca2NH1–xex– to Ru, (ii) the formation of NHx species promoted at the Ru/Ca2NH1–xex– interface, and (iii) hydrogen poisoning suppression of Ru by fast hydrogen migration at the Ru/Ca2NH1–xex– interface. Microkinetic modeling also revealed that NH3 formation (NH2 + H → NH3) at the Ru/Ca2NH1–xex– interface is the RDS. These findings are consistent with the experimental results and validate the reaction mechanism dealt with in this research.
中文翻译:

钌负载氢化物催化氨合成机理的第一性原理和微动力学研究
负载钌的氢化物是在温和条件下合成氨(NH 3)的有效催化剂。金属氢化物,例如Ca 2 NH,具有在氢空位处的表面阴离子电子(Ca 2 NH 1– x e x –),可以很好地用作Ru的活性催化载体材料。所得的催化剂对于NH 3合成表现出良好的性能,其表观活化能大大降低,并且抑制了Ru的氢中毒。然而,从微观的观点来看,反应机理和速率确定步骤(RDS)尚未阐明。在这里,我们成功地再现了NH 3的实验结果通过使用密度泛函理论(DFT)计算的微动力学建模进行合成。确定了三个基本机制:(i)通过从Ca 2 NH 1– x e x –注入电子来促进氮裂解为Ru,(ii)在Ru / Ca 2 NH 1– x处促进了NH x物种的形成。e x –界面,以及(iii)通过Ru / Ca 2 NH 1– x e x –界面处的快速氢迁移来抑制Ru的氢中毒。微观动力学模型还表明,NH 3的形成(NH 2Ru / Ca 2 NH 1– x e x –界面上的+ H→NH 3)是RDS。这些发现与实验结果相吻合,并验证了本研究中涉及的反应机理。
更新日期:2020-01-10
中文翻译:

钌负载氢化物催化氨合成机理的第一性原理和微动力学研究
负载钌的氢化物是在温和条件下合成氨(NH 3)的有效催化剂。金属氢化物,例如Ca 2 NH,具有在氢空位处的表面阴离子电子(Ca 2 NH 1– x e x –),可以很好地用作Ru的活性催化载体材料。所得的催化剂对于NH 3合成表现出良好的性能,其表观活化能大大降低,并且抑制了Ru的氢中毒。然而,从微观的观点来看,反应机理和速率确定步骤(RDS)尚未阐明。在这里,我们成功地再现了NH 3的实验结果通过使用密度泛函理论(DFT)计算的微动力学建模进行合成。确定了三个基本机制:(i)通过从Ca 2 NH 1– x e x –注入电子来促进氮裂解为Ru,(ii)在Ru / Ca 2 NH 1– x处促进了NH x物种的形成。e x –界面,以及(iii)通过Ru / Ca 2 NH 1– x e x –界面处的快速氢迁移来抑制Ru的氢中毒。微观动力学模型还表明,NH 3的形成(NH 2Ru / Ca 2 NH 1– x e x –界面上的+ H→NH 3)是RDS。这些发现与实验结果相吻合,并验证了本研究中涉及的反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号