当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel 3-[4-alkoxy-3-(1H-tetrazol-1-yl) phenyl]-1,2,4-oxadiazol-5(4H)-ones as promising xanthine oxidase inhibitors: Design, synthesis and biological evaluation.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.bioorg.2019.103564 Jun Gao 1 , Zhaofeng Zhang 1 , Bing Zhang 1 , Qing Mao 1 , Xiwen Dai 1 , Qian Zou 2 , Yu Lei 1 , Yao Feng 1 , Shaojie Wang 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-12-31 , DOI: 10.1016/j.bioorg.2019.103564 Jun Gao 1 , Zhaofeng Zhang 1 , Bing Zhang 1 , Qing Mao 1 , Xiwen Dai 1 , Qian Zou 2 , Yu Lei 1 , Yao Feng 1 , Shaojie Wang 1
Affiliation
|
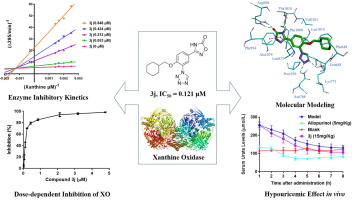
Xanthine oxidase (XO) is a critical target for the therapy of hyperuricemia and gout. In this study, a number of 3-[4-alkoxy-3-(1H-tetrazol-1-yl) phenyl]-1,2,4-oxadiazol-5(4H)-ones (3a-3w) were newly designed by a bioisosteric replacement and hybrid strategy with the hope of obtaining novel and effective nonpurine XO inhibitors. Subsequently, these compounds were synthesized through a three-step procedure, with good yields. In addition, the in vitro bovine XO inhibitions were measured by spectrophotometric determination of uric acid formation at 295 nm using allopurinol as a positive control. As a result, compound 3j was found to be the most potent XO inhibitor, with an IC50 value of 0.121 µM, which was approximately 63-fold more potent than allopurinol, and the analysis of the structure-activity relationships indicated that the hydrophobic group at 4'-position was essential for inhibitory potency. Additionally, the molecular modeling results showed that the 1,2,4-oxadiazol-5(4H)-one moiety binds to XO active site via various hydrogen bonds with Arg880 and Thr1010. Moreover, the compound 3j was demonstrated to be a mixed-type nonpurine XO inhibitor. Furthermore, the hypouricemic studies on a rat model, induced by potassium oxonate, demonstrated that serum uric acid levels could be effectually reduced by compound 3j at an oral dose of 15 mg/kg. Therefore, compound 3j could be a promising lead compound for the treatment of hyperuricemia and gout.
中文翻译:

新型的3- [4-烷氧基-3-(1H-四唑-1-基)苯基] -1,2,4-恶二唑-5(4H)-作为黄嘌呤氧化酶抑制剂:设计,合成和生物学评估。
黄嘌呤氧化酶(XO)是治疗高尿酸血症和痛风的关键靶标。在这项研究中,新设计了一些3- [4-烷氧基-3-(1H-四唑-1-基)苯基] -1,2,4-恶二唑-5(4H)-一(3a-3w)通过生物等位替代和混合策略,希望获得新型有效的非嘌呤XO抑制剂。随后,通过三步法合成了这些化合物,收率很高。另外,使用别嘌呤醇作为阳性对照,通过分光光度法测定在295 nm处的尿酸形成来测量体外牛XO抑制作用。结果,发现化合物3j是最有效的XO抑制剂,IC50值为0.121 µM,比别嘌呤醇的效价高约63倍,结构-活性关系的分析表明,在4'-位的疏水基团对于抑制效能是必不可少的。此外,分子建模结果表明,1,2,4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。
更新日期:2019-12-31
中文翻译:

新型的3- [4-烷氧基-3-(1H-四唑-1-基)苯基] -1,2,4-恶二唑-5(4H)-作为黄嘌呤氧化酶抑制剂:设计,合成和生物学评估。
黄嘌呤氧化酶(XO)是治疗高尿酸血症和痛风的关键靶标。在这项研究中,新设计了一些3- [4-烷氧基-3-(1H-四唑-1-基)苯基] -1,2,4-恶二唑-5(4H)-一(3a-3w)通过生物等位替代和混合策略,希望获得新型有效的非嘌呤XO抑制剂。随后,通过三步法合成了这些化合物,收率很高。另外,使用别嘌呤醇作为阳性对照,通过分光光度法测定在295 nm处的尿酸形成来测量体外牛XO抑制作用。结果,发现化合物3j是最有效的XO抑制剂,IC50值为0.121 µM,比别嘌呤醇的效价高约63倍,结构-活性关系的分析表明,在4'-位的疏水基团对于抑制效能是必不可少的。此外,分子建模结果表明,1,2,4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。4-恶二唑-5(4H)-one部分通过与Arg880和Thr1010的各种氢键结合到XO活性位。此外,化合物3j被证明是混合型非嘌呤XO抑制剂。此外,由草酸钾诱导的对大鼠模型的降尿酸研究表明,口服剂量为15 mg / kg的化合物3j可有效降低血清尿酸水平。因此,化合物3j可能是治疗高尿酸血症和痛风的有前途的先导化合物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号