当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Robust Model for Prediction of U(VI) Adsorption onto Ferrihydrite Consistent with Spectroscopic Observations.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.est.9b06556 Yui Kobayashi 1 , Keisuke Fukushi 2 , Shigeyori Kosugi 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-04 , DOI: 10.1021/acs.est.9b06556 Yui Kobayashi 1 , Keisuke Fukushi 2 , Shigeyori Kosugi 3
Affiliation
|
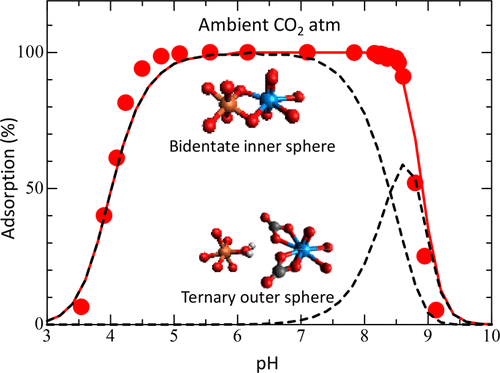
A robust model that can predict the adsorption behavior of U(VI) on ferrihydrite under a wide range of environmental conditions was developed with the aid of an extended triple-layer model. X-ray absorption spectroscopic observations from previous studies showed that the predominant U(VI) surface species on ferrihydrite was commonly a bidentate inner-sphere species under ambient CO2 conditions. Previous surface complexation models, however, could not predict U(VI) surface speciation because of the lack of sufficient macroscopic adsorption datasets with which to estimate the surface complexation reaction. In this study, we obtained U(VI) adsorption data at U(VI) concentrations of 10nM under a wide range of pH, ionic strength, and solid concentrations in NaNO3 solutions with/without atmospheric CO2. We determined the stoichiometries of the U(VI) adsorption reactions and the equilibrium constants with the adsorption data and the U(VI) hydroxyl constants recently estimated from direct luminescence measurements. A single set of equilibrium constants for the reactions could reproduce reasonably well the reported adsorption datasets obtained under a wide range of pH values (2-12), U(VI) concentrations (10-8 to 10-4 M), ionic strengths (0.004-0.5), and CO2 partial pressures (<10-6 to 10-1.7 atm). The model could also predict all U(VI) surface speciation consistent with previous spectroscopic observations under a wide range of solution conditions.
中文翻译:

与光谱观察结果一致的鲁棒模型,用于预测U(VI)吸附到三水铁矿上。
借助扩展的三层模型,开发了一个可以预测U(VI)在水铁矿上广泛环境条件下的吸附行为的鲁棒模型。先前研究的X射线吸收光谱观察表明,在环境CO2条件下,水铁矿上主要的U(VI)表面物种通常是双齿内球物种。但是,由于缺乏足够的宏观吸附数据集来估计表面络合反应,以前的表面络合模型无法预测U(VI)表面形态。在这项研究中,我们获得了在10nM的U(VI)浓度下,在宽范围的pH,离子强度和含或不含大气CO2的NaNO3溶液中固体浓度下的U(VI)吸附数据。我们确定了U(VI)吸附反应的化学计量和平衡常数,以及最近通过直接发光测量估算的吸附数据和U(VI)羟基常数。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。
更新日期:2020-02-06
中文翻译:

与光谱观察结果一致的鲁棒模型,用于预测U(VI)吸附到三水铁矿上。
借助扩展的三层模型,开发了一个可以预测U(VI)在水铁矿上广泛环境条件下的吸附行为的鲁棒模型。先前研究的X射线吸收光谱观察表明,在环境CO2条件下,水铁矿上主要的U(VI)表面物种通常是双齿内球物种。但是,由于缺乏足够的宏观吸附数据集来估计表面络合反应,以前的表面络合模型无法预测U(VI)表面形态。在这项研究中,我们获得了在10nM的U(VI)浓度下,在宽范围的pH,离子强度和含或不含大气CO2的NaNO3溶液中固体浓度下的U(VI)吸附数据。我们确定了U(VI)吸附反应的化学计量和平衡常数,以及最近通过直接发光测量估算的吸附数据和U(VI)羟基常数。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。一组反应的平衡常数可以很好地重现在宽范围的pH值(2-12),U(VI)浓度(10-8至10-4 M),离子强度( 0.004-0.5)和CO2分压(<10-6至10-1.7 atm)。该模型还可以预测在宽范围的溶液条件下与以前的光谱观察结果一致的所有U(VI)表面形态。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号