当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics and Kinetics of Click Reaction between Benzyl Azide and Different Alkynes by Microcalorimetry
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.oprd.9b00435 Xiaoyi Li 1 , Bo Jin 1 , Zhicheng Guo 1 , Shijin Chu 1 , Rufang Peng 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-01-22 , DOI: 10.1021/acs.oprd.9b00435 Xiaoyi Li 1 , Bo Jin 1 , Zhicheng Guo 1 , Shijin Chu 1 , Rufang Peng 1
Affiliation
|
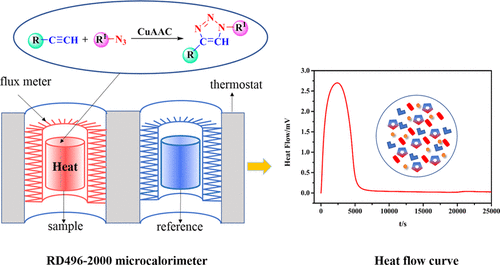
The copper(I)-catalyzed 1,3-dipolar cycloaddition of azides and alkynes provides diverse building blocks for chemical synthesis, biochemistry, pharmaceutical industries, and materials science. To provide a good theoretical support for industrial amplification reaction, it is significant to study the catalytic properties and the corresponding kinetics of a given reaction. In this article, the reaction kinetics and thermodynamics of “click reaction” between benzyl azide and different alkynes were investigated by microcalorimetry. Benzyl azide and ethyl propiolate were used as model reactions. The effects of a variety of parameters were studied in a calorimeter to establish the kinetics and mechanism of the reaction under isothermal conditions. The activation energies for the reaction of benzyl azide with 4-nitrophenylacetylene, ethyl propiolate, and 3-butyn-2-one were 22.99 ± 0.13, 55.81 ± 0.74, and 56.75 ± 0.65 kJ mol–1, respectively. The difference of reaction kinetics of different alkynes was explained by the reaction mechanism combined with theoretical calculations, and the reaction is intrinsically kinetically controlled.
中文翻译:

微量热法测定叠氮化苄与不同炔烃的点击反应的热力学和动力学
铜(I)催化的叠氮化物和炔烃的1,3-偶极环加成反应为化学合成,生物化学,制药工业和材料科学提供了多种构建基块。为了为工业扩增反应提供良好的理论支持,研究给定反应的催化性能和相应的动力学具有重要意义。在本文中,通过微量量热法研究了叠氮化苄与不同炔烃之间“点击反应”的反应动力学和热力学。叠氮化苄和丙酸乙酯用作模型反应。在量热仪中研究了各种参数的影响,以建立等温条件下反应的动力学和机理。叠氮化苄与4-硝基苯基乙炔,丙酸乙酯,分别为–1。通过反应机理结合理论计算解释了不同炔烃反应动力学的差异,反应是内在动力学控制的。
更新日期:2020-01-23
中文翻译:

微量热法测定叠氮化苄与不同炔烃的点击反应的热力学和动力学
铜(I)催化的叠氮化物和炔烃的1,3-偶极环加成反应为化学合成,生物化学,制药工业和材料科学提供了多种构建基块。为了为工业扩增反应提供良好的理论支持,研究给定反应的催化性能和相应的动力学具有重要意义。在本文中,通过微量量热法研究了叠氮化苄与不同炔烃之间“点击反应”的反应动力学和热力学。叠氮化苄和丙酸乙酯用作模型反应。在量热仪中研究了各种参数的影响,以建立等温条件下反应的动力学和机理。叠氮化苄与4-硝基苯基乙炔,丙酸乙酯,分别为–1。通过反应机理结合理论计算解释了不同炔烃反应动力学的差异,反应是内在动力学控制的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号